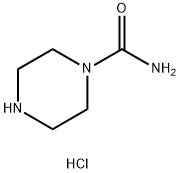
PIPERAZINE-1-CARBOXYLIC ACID AMIDE HCL synthesis
- Product Name:PIPERAZINE-1-CARBOXYLIC ACID AMIDE HCL
- CAS Number:474711-89-2
- Molecular formula:C5H12ClN3O
- Molecular Weight:165.62

883554-88-9

474711-89-2
General procedure for the synthesis of piperazine-1-amide hydrochloride from tert-butyl 4-carbamoylpiperazine-1-carboxylate: To a dichloromethane (DCM, 2 mL) solution of tert-butyl 4-carbamoylpiperazine-1-carboxylate (0.16 g, 0.7 mmol) was added an ethyl acetate (EtOAc) solution of hydrochloric acid (4 M, 2 mL). The reaction mixture was stirred at room temperature for 30 min and then concentrated under reduced pressure to give piperazine-1-amide hydrochloride as a white solid (0.16 g, 100% yield). The product was characterized by 1H NMR (600 MHz, CD3OD): δ 3.71-3.73 (m, 4H), 3.25-3.27 (m, 4H); MS-ESI: m/z 130.10 [M + H - HCl]+.

883554-88-9
33 suppliers
inquiry

474711-89-2
61 suppliers
$21.00/100mg
Yield:474711-89-2 100%
Reaction Conditions:
with hydrogenchloride in dichloromethane;ethyl acetate at 20; for 0.5 h;Inert atmosphere;
Steps:
45.1 Step 1) : piperazine-1-carboxamide hydrochloride
To a solution of tert-butyl4-carbamoylpiperazine-1-carboxylate (0.16 g, 0.7 mmol) in DCM (2 mL) was addeda HCl in EtOAc solution (4 M, 2 mL) . The mixture was stirred at rt for 30 min andconcentrated to give the title compound as a white solid (0.16 g, 100) . 1H NMR (600 MHz, CD3OD): δ ppm 3.71-3.73 (m, 4H) , 3.25-3.27 (m, 4H) and MS-ESI: m/z 130.10[M+H-HCl] + .
References:
WO2016/34134,2016,A1 Location in patent:Paragraph 00403