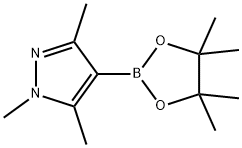
1,3,5-TRIMETHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOLE synthesis
- Product Name:1,3,5-TRIMETHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOLE
- CAS Number:844891-04-9
- Molecular formula:C12H21BN2O2
- Molecular Weight:236.12
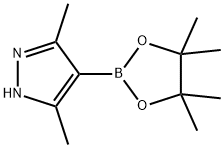
857530-80-4

74-88-4

844891-04-9
General procedure for the synthesis of 1,3,5-trimethyl-1H-pyrazole-4-boronic acid pinacol ester from 3,5-dimethylpyrazole-4-boronic acid pinacol ester and iodomethane: 3,5-dimethyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (500 mg, 2.25 mmol) was suspended in acetone (5 mL) in acetone (5 mL), potassium carbonate (622 mg, 4.5 mmol) and iodomethane (0.21 mL, 3.37 mmol) were added. The reaction mixture was heated to 60 °C and kept for 4.5 hours. After completion of the reaction, it was cooled to room temperature, water was added, the aqueous phase was separated and extracted three times with ethyl acetate (EtOAc). The organic phases were combined, washed with water, dried over anhydrous magnesium sulfate (MgSO4), and concentrated in vacuo to afford 1,3,5-trimethyl-1H-pyrazole-4-boronic acid pinacol ester (403 mg, 76%) as a light yellow oil.LCMS (ES+) m/z 237 (M+H)+, retention time (RT) 3.35 min (Method 1).
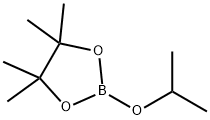
61676-62-8
325 suppliers
$10.00/5g

844891-04-9
149 suppliers
$7.00/250mg
Yield: 77%
Reaction Conditions:
Stage #1:4-bromo-1,3,5-trimethylpyrazole with n-butyllithium in tetrahydrofuran;hexane at -78 - 40; for 0.333333 h;
Stage #2:2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at -78 - 40;
Steps:
21.21A
Example 21; 1,3,5-Trimethyl-4-{4-[3-(2-methyl-pyrrolidin-1-yl)-trans-cyclobutyl]-phenyl}-1H-pyrazole; Example 21A; 1,3,5-Trimethyl-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H-pyrazole; A solution of 4-bromo-1,3,5-trimethyl-1H-pyrazole (1 g, 5.3 mmol) in anhydrous THF (20 mL) cooled to -78° C. under a nitrogen atmosphere was treated dropwise with n-butyl lithium (4.2 mL, 1.6 M in hexane) and stirred at room temperature for 20 minutes. Then, 2-isopropoxy-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (1.7 mL, 8.3 mmol) was added dropwise at -78° C. and allowed to warm to ambient temperature overnight. Ethyl acetate was added and the mixture was filtered through diatomaceous earth. The filtrate was concentrated and chromatographed on silica gel eluting with 40% ethyl acetate in hexanes to provide the title compound as white crystals (996 mg, 77%). 1H NMR (300 MHz, CDCl3) δ 1.29 (s, 12H), 2.33 (s, 3H), 2.37 (s, 3H), 3.69 (s, 3H); (DCl/NH3) m/z 237 (M+H)+.
References:
Liu, Huaqing;Hancock, Arthur A.;Cowart, Marlon D.;Hancock, Kathryn J. US2007/78133, 2007, A1 Location in patent:Page/Page column 29-30

857530-80-4
143 suppliers
$9.00/250mg

74-88-4
358 suppliers
$15.00/10g

844891-04-9
149 suppliers
$7.00/250mg
76-09-5
396 suppliers
$8.19/250mg

847818-62-6
63 suppliers
$32.00/100mg

844891-04-9
149 suppliers
$7.00/250mg
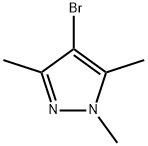
15801-69-1
147 suppliers
$10.00/5g
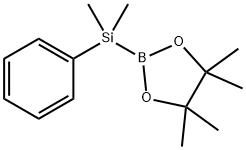
185990-03-8
91 suppliers
$17.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

844891-04-9
149 suppliers
$7.00/250mg
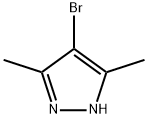
3398-16-1
192 suppliers
$7.00/5g

844891-04-9
149 suppliers
$7.00/250mg