
Boronic acid, B-(1,3,5-trimethyl-1H-pyrazol-4-yl)- synthesis
- Product Name:Boronic acid, B-(1,3,5-trimethyl-1H-pyrazol-4-yl)-
- CAS Number:847818-62-6
- Molecular formula:C6H11BN2O2
- Molecular Weight:153.97
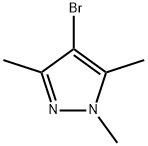
15801-69-1

847818-62-6
The general procedure for the synthesis of (1,3,5-trimethyl-1H-pyrazol-4-yl)boronic acid from 4-bromo-1,3,5-trimethylpyrazole was as follows: to a dry flask was added 300 mg (1.59 mmol) of 4-bromo-1,3,5-trimethylpyrazole and 4.0 mL of THF. The mixture was cooled to -78°C, and 1 equivalent of n BuLi (1.6 M in hexane). After stirring the reaction mixture for 1.5 hours, 0.19 mL (1.08 equiv) of trimethyl borate was added. Stirring was continued for 2 hr while allowing the reaction bath temperature to slowly increase to -10°C. Subsequently, 1.5 mL of 5N HCl was added and stirring was continued for 30 min. Upon completion of the reaction, the aqueous layer was extracted three times with ethyl acetate. The organic phases were combined, dried with anhydrous MgSO4, filtered and concentrated under reduced pressure to an oil. The oil was dissolved in a solvent mixture of methanol/dichloromethane and concentrated again under reduced pressure. The residue was ground with acetone/ethyl acetate mixed solvent and filtered to give (1,3,5-trimethyl-1H-pyrazol-4-yl)boronic acid as a white solid in a yield of 92.1 mg (37.6% yield).1H-NMR (DMSO-d6) δ: 5.92 (s, 1H), 3.72 (s, 3H), 2.34 (s, 3H), 2.26 (s, 3H ) ppm.

15801-69-1
147 suppliers
$10.00/5g

847818-62-6
63 suppliers
$32.00/100mg
Yield:847818-62-6 37.6%
Reaction Conditions:
Stage #1: 4-bromo-1,3,5-trimethylpyrazolewith n-butyllithium in tetrahydrofuran at -78; for 1.5 h;
Stage #2: with Trimethyl borate in tetrahydrofuran at -10; for 2 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran at -10; for 0.5 h;
Steps:
98.A
Example 98.; Preparation of 8-(1-ethyl-propyl)-2,6-dimethyl-3-(l,3,5-trimethyl-7H-pyrazol-4-yl)- imidazo[1,2-b]pyridazine.; A. l,3,5-Trimethylpyrazole-4-boronic acid.; To a dry flask is added 300 mg (1.59 mmol) of 4-bromo-l,3,5-trimethyl pyrazole to 4.0 ml THF. The mixture is cooled to -78°C and leq of n-BuLi (1.6 M) is added via syringe. The mixture is stirred 1.5 hrs, and 0.19ml of trimethylborate (1.08 eq) is added. The reaction mixture is stirred 2 hrs, allowing bath to reach -10°C, then 1.5 ml of 5N HCl is added and stirred 30 minutes longer. The aqueous layer is extracted 3 times with ethyl acetate. The combined organics are dried over MgSO4, filtered, and evaporated to an oil. The oil is dissolved in methanol/methylene chloride and re-evaporated. The residue is triturated with acetone/ethyl acetate then filtered to obtain title compound as a white solid 92.1 mg (37.6%). 1H-NMR (DMSOd6): £5.92 (s); 3.72 (s, 3H); 2.34 (s, 3H); 2.26 (s, 3H) ppm.
References:
WO2006/102194,2006,A1 Location in patent:Page/Page column 95
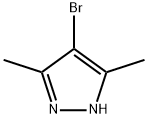
3398-16-1
192 suppliers
$7.00/5g

847818-62-6
63 suppliers
$32.00/100mg