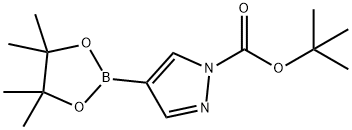
1-Boc-pyrazole-4-boronic acid pinacol ester synthesis
- Product Name:1-Boc-pyrazole-4-boronic acid pinacol ester
- CAS Number:552846-17-0
- Molecular formula:C14H23BN2O4
- Molecular Weight:294.15

269410-08-4

24424-99-5

552846-17-0
4-Pyrazoleboronic acid pinacol ester (1.16 g, 6.0 mmol) and di-tert-butyl dicarbonate (1.58 g, 7.2 mmol) were used as raw materials and dissolved in dichloromethane (40 mL). A catalytic amount of 4-dimethylaminopyridine (102 mg, 0.84 mmol) was added and the reaction was stirred at room temperature for 4 hours. After completion of the reaction, water was added to the reaction system and extracted with ethyl acetate. The organic phase was washed with saturated aqueous sodium chloride solution and then the solvent was removed by rotary evaporation to give the crude product 1-Boc-pyrazole-4-boronic acid pinacol ester (1.5 g, 85% yield).

269410-08-4
398 suppliers
$5.00/1g

24424-99-5
865 suppliers
$13.50/25G

552846-17-0
236 suppliers
$11.00/1g
Yield:552846-17-0 99.68%
Reaction Conditions:
with dmap;N-ethyl-N,N-diisopropylamine in dichloromethane at 20;
Steps:
2.1; 9.1 Step 1:
Compound Reg-1-16-a (4.5 g, 2.58 mmol) and dichloromethane (200 mL) were added to a 100 mL flask, diisopropylethylamine (5.99 g, 46.38 mmol) and 4-dimethylaminopyridine (424 mg, 3.48 mmol) were added, followed by slow dropwise addition of Boc2O (7.59 g, 34.79 mmol). The reaction was performed overnight at room temperature. Thin layer chromatography (petroleum ether : ethyl acetate=3:1) indicated the reaction was complete. The reaction solution was concentrated to give a crude product, which was dissolved in dichloromethane (100 mL), and the organic phase was then washed three times with 0.5M dilute hydrochloric acid. The organic phase was further washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain Reg-1-16-b (6.8 g, colorless oil, yield: 99.68%). MS m/z (ESI): 195.2 [M-Boc+H].
References:
Beijing Tide Pharmaceutical Co., Ltd.;Zhao, Yanping;Wang, Hongjun;Li, Gong;Jiang, Yuanyuan;Li, Xiang;Zhou, Liying;Liu, Yanan EP3421464, 2019, A1 Location in patent:Paragraph 0109; 0110; 0187; 0188
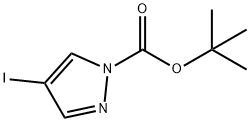
121669-70-3
108 suppliers
$8.00/250mg
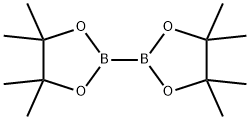
73183-34-3
587 suppliers
$6.00/5g

552846-17-0
236 suppliers
$11.00/1g
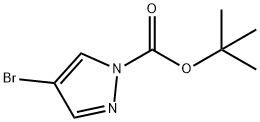
1150271-23-0
76 suppliers
$10.00/1g

73183-34-3
587 suppliers
$6.00/5g

552846-17-0
236 suppliers
$11.00/1g
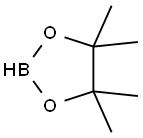
25015-63-8
223 suppliers
$22.39/5G
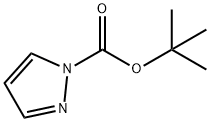
219580-32-2
90 suppliers
$9.00/1g

552846-17-0
236 suppliers
$11.00/1g

269410-08-4
398 suppliers
$5.00/1g

552846-17-0
236 suppliers
$11.00/1g