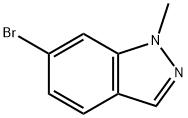
6-Bromo-1-methyl-1H-indazole synthesis
- Product Name:6-Bromo-1-methyl-1H-indazole
- CAS Number:590417-94-0
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
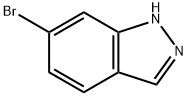
79762-54-2

74-88-4

590417-94-0
6-Bromo-1H-indazole (400 mg) was dissolved in methanol (10 mL). Potassium hydroxide (450 mg) and iodomethane (0.50 mL) were added sequentially to this solution and the reaction mixture was heated to reflux for 2.5 hours. After completion of the reaction, it was cooled to room temperature, diluted with ether, washed sequentially with water and saturated saline, and the organic phase was dried over anhydrous sodium sulfate and concentrated. The target product 6-bromo-1-methylindazole (160 mg) and its isomers were isolated by Combiflash fast column chromatography.
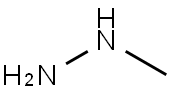
60-34-4
0 suppliers
$51.79/25g

57848-46-1
388 suppliers
$9.00/1g

590417-94-0
170 suppliers
$8.00/250mg
Yield: 85%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl acetamide;water at 100; for 40 h;Industrial scale;Reagent/catalyst;Temperature;Solvent;
Steps:
1.1; 2.1; 3.1; 4-9; 10.1
(1) Add 3000.0 ml of N,N-dimethylacetamide to the reaction flask.2-fluoro-4-bromobenzaldehyde 1500.0 g (7.39 mol, 1.00 equ.), cooled to 0 ° C,Add 1530.0 g of potassium carbonate (11.10 mol, 1.50 equ.), and then add 3000.0 ml of methyl hydrazine (40 wt.% aqueous solution) dropwise. After the addition, the temperature was raised to 100 ° C, and the reaction was carried out for 40 hours.The raw material was completely reacted, cooled to room temperature, extracted with ethyl acetate and water, and layered.The organic phase was washed with 2M aqueous hydrochloric acid solution and 7% aqueous sodium hydrogencarbonate solution and brine, and the organic phase was concentrated, n-heptane crystals, filtered, and the cake was dried at 40 ° C for 22 hours.Obtaining 1320.0 g of 6-bromo-1-methylcarbazole,Yield: 85%.
References:
Shaoyuan Science And Technology (Shanghai) Co., Ltd.;Qidong Shaoyuan Chemical Technology Co., Ltd.;Yang Jun;Xue Duoqing;Wu Yong;Chen Lihuang;Zhai Lianhua CN110128347, 2019, A Location in patent:Paragraph 0046-0049; 0055-0057; 0064-0066; 0073-0084; 0089

79762-54-2
380 suppliers
$3.00/1g

74-88-4
356 suppliers
$15.00/10g

590417-94-0
170 suppliers
$8.00/250mg

74-88-4
356 suppliers
$15.00/10g

590417-94-0
170 suppliers
$8.00/250mg

590417-95-1
123 suppliers
$9.00/250mg

79762-54-2
380 suppliers
$3.00/1g

74-88-4
356 suppliers
$15.00/10g

590417-94-0
170 suppliers
$8.00/250mg

590417-95-1
123 suppliers
$9.00/250mg

39478-78-9
324 suppliers
$10.00/5g

590417-94-0
170 suppliers
$8.00/250mg