
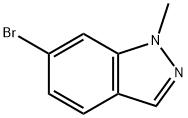
6-BROMO-2-METHYL-2H-INDAZOLE synthesis
- Product Name:6-BROMO-2-METHYL-2H-INDAZOLE
- CAS Number:590417-95-1
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06

74-88-4
590417-94-0

590417-95-1
Example 402 Part A: To a solution of tetrahydrofuran (THF) (50 mL) of 6-bromoindazole (402A) (5.0 g, 25.4 mmol) was slowly added sodium hydride (95%, 672 mg, 26.6 mmol) under ice bath cooling conditions. The reaction mixture was stirred for 30 min. Subsequently, iodomethane (6.36 mL, 102 mmol) was added dropwise at room temperature. Upon completion of the reaction, the reaction was quenched with aqueous ammonium chloride and the organic and aqueous layers were separated. The aqueous layer was extracted with ethyl acetate and the combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Purification by silica gel column chromatography (eluent: ethyl acetate/hexane gradient) afforded 1-methyl-6-bromoindazole (402B) (2.71 g, 51% yield) as a yellow oil and 2-methyl-6-bromoindazole (402C) (2.28 g, 43% yield) as a yellow crystalline solid, respectively. HPLC-MS analysis of 402B showed that the retention time t R = 1.69 min (UV 254 nm); calculated molecular weight C8H7BrN2 was 209.98, measured LCMS m/z 211.0 ([M + H]+). 402C was analyzed by HPLC-MS: retention time t R = 1.54 min (UV 254 nm); calculated molecular weight C8H7BrN2 was 209.98, measured LCMS m/z 211.0 ([M + H]+). 211.0 ([M + H]+).

74-88-4
356 suppliers
$15.00/10g

590417-94-0
170 suppliers
$8.00/250mg

590417-95-1
123 suppliers
$9.00/250mg
Yield:590417-94-0 51% ,590417-95-1 43%
Reaction Conditions:
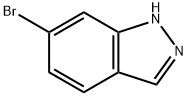
Stage #1: 6-bromo-1H-indazolewith sodium hydride in tetrahydrofuran at 0; for 0.5 h;
Stage #2: methyl iodide at 20;
Steps:
402.A
Example 402 Part A: To 6-bromoindazole (402A) (5.0 g, 25.4 mmol) in THF (50 mL) was added sodium hydride (95%, 672 mg, 26.6 mmol) with ice bath cooling. The mixture was stirred for 30 minutes. Methyl iodide (6.36 mL, 102 mmol) was added at room temperature. The reaction mixture was quenched with ammonium chloride solution and the layers were separated. The aqueous layer was extracted with ethyl acetate. The combined organic layer was washed with brine, dried over sodium sulfate and concentrated. Purification by column chromatography (SiO2, ethyl acetate/hexane gradient) afforded 1-methyl-6-bromoindazole (402B) (2.71 g, 51%) as a yellow oil and 2-methyl-6- bromoindazole (402C) (2.28 g, 43%) as a yellow crystalline solid. 402B: HPLC-MS tR = 1.69 min (UV254 nm); mass calculated for formulaC8H7BrN2209.98, observed LCMS m/z 211.0 (M+H).402C: HPLC-MS tR = 1.54 min (UV254 nm); mass calculated for formulaC8H7BrN2209.98, observed LCMS m/z 211.0 (M+H).
References:
WO2007/84451,2007,A1 Location in patent:Page/Page column 142
79762-54-2
380 suppliers
$3.00/1g

74-88-4
356 suppliers
$15.00/10g

590417-95-1
123 suppliers
$9.00/250mg

79762-54-2
380 suppliers
$3.00/1g

74-88-4
356 suppliers
$15.00/10g

590417-94-0
170 suppliers
$8.00/250mg

590417-95-1
123 suppliers
$9.00/250mg