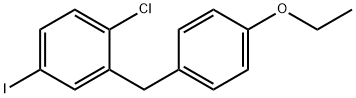
1-Chloro-2-(4-ethoxybenzyl)-4-iodobenzene synthesis
- Product Name:1-Chloro-2-(4-ethoxybenzyl)-4-iodobenzene
- CAS Number:1103738-29-9
- Molecular formula:C15H14ClIO
- Molecular Weight:372.63
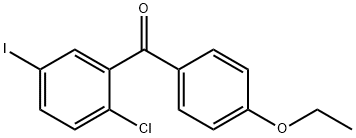
1103738-26-6

1103738-29-9
Under nitrogen protection, 1 g of (2-chloro-5-iodophenyl)(4-ethoxyphenyl)methanone and 10.88 g of dichloromethane were added to a 100 mL reaction flask and heated to 30-40 °C. Subsequently 2.88 g of acetonitrile and 0.2 g of sodium borohydride were added and the reaction was stirred at 30-40 °C for 0.5 hours. Slowly 0.84 g of trimethylchlorosilane was added dropwise and the temperature was maintained at 30-40 °C for 4 hours of reaction. After that, 0.6 g of sodium borohydride and 2.52 g of trimethylchlorosilane were added and the reaction was continued at 30-40°C for 4 hours. The reaction solution was cooled to 0-5 °C, 2.2 g of boron trifluoride diethyl ether was added slowly dropwise, the temperature was controlled to be lower than 10 °C, and then the temperature was raised to 30-35 °C and maintained for 4 hours. Cooled again to 10-15 °C, added 0.6 g of sodium borohydride and 1.65 g of boron trifluoride diethyl ether, and heated to 30-35 °C for 4 hours. After the reaction was completed, cooled to 0-5 °C, 20 mL of dichloromethane was added, 20 mL of 12% sodium carbonate solution was added slowly dropwise, and stirring was continued for 20 minutes after the drop. The liquid was separated and the aqueous phase was extracted twice with 40 mL of dichloromethane. The organic phases were combined, washed with water once, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain a light yellow oily crude product. The crude product was dissolved in 3mL of toluene, heated to 60°C, slowly added 12mL of methanol dropwise, cooled to -10°C and crystallized for 8 hours. Filtering, drying, to get 0.85g white solid product, yield 86%.

1103738-26-6
122 suppliers
$18.00/100mg

1103738-29-9
286 suppliers
$8.00/250mg
Yield:1103738-29-9 97.4%
Reaction Conditions:
with triethylsilane;boron trifluoride diethyl etherate in acetonitrile at 5 - 20; for 17.5 h;
Steps:
6.3 6.3.
A jacketed 1 L three-necked round bottom flask with mechanical stirrer, rubber septum with temperature probe and gas bubbler was charged with crude (2-chloro-5-iodophenyl)(4-ethoxyphenyl)methanone (30.13 g, 77.93 mmol), acetonitrile (300 mL, KF=0.004 wt % water) and the suspension was set stirring under nitrogen and was cooled to about 5° C. Then triethylsilane (28 mL, 175.30 mmol, 2.25 equiv) was added followed by boron trifluoride-diethyletherate (24 mL, 194.46 mmol, 2.50 equiv) which was added over about 30 seconds. The reaction was warmed to ambient over 30 min and was stirred for 17 hours. The reaction was diluted with methyl tert-butyl ether (150 mL) followed by saturated aq sodium bicarbonate (150 mL) which was added over about 1 minutes. Mild gas evolution was noticed and the biphasic solution was stirred at ambient for 45 minutes. The upper organic phase was washed with saturated aq. sodium bicarbonate (100 mL), and with saturated aq. sodium chloride (50 mL). The washed extract was concentrated on a rotary evaporator to about one half of its original volume and was diluted with water (70 mL). Further concentration in vacuo at 45° C. was done until white prills formed which were allowed to cool to ambient while stirring. After about 30 minutes at ambient, the suspended solids were isolated by filtration, washed with water (30 mL), and were dried in vacuo at 45° C. After about 2.5 hours, this afforded 1-chloro-2-(4-ethoxybenzyl)-4-iodobenzene as a slightly waxy white granular powder (28.28 g, 98.2 area % by HPLC at 220 nm, 97.4 % “as-is” yield).
References:
US2009/30198,2009,A1 Location in patent:Page/Page column 9
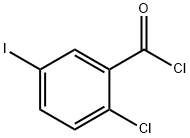
281652-58-2
24 suppliers
inquiry
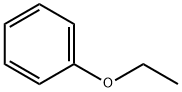
103-73-1
361 suppliers
$8.00/25g

1103738-29-9
286 suppliers
$8.00/250mg

793695-85-9
50 suppliers
$116.00/250mg
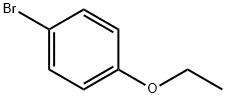
588-96-5
300 suppliers
$7.00/5g

1103738-29-9
286 suppliers
$8.00/250mg
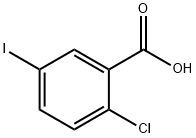
19094-56-5
540 suppliers
$6.00/5g

1103738-29-9
286 suppliers
$8.00/250mg

281652-58-2
24 suppliers
inquiry

1103738-29-9
286 suppliers
$8.00/250mg