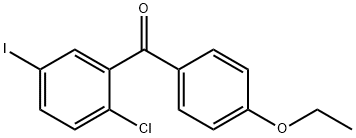
(2-Chloro-5-iodophenyl)(4-ethoxyphenyl)methanone synthesis
- Product Name:(2-Chloro-5-iodophenyl)(4-ethoxyphenyl)methanone
- CAS Number:1103738-26-6
- Molecular formula:C15H12ClIO2
- Molecular Weight:386.61
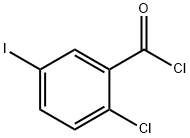
281652-58-2
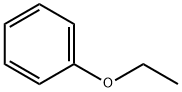
103-73-1

1103738-26-6
A 2L three-necked round-bottomed flask with a mechanical stirrer (equipped with a jacket, a rubber septum with a temperature probe, and a pressure-equalizing charging funnel with a gas bubbler) was filled with aluminum chloride (97.68 g, 0.733 mol, 1.04 eq.) and methylene chloride (0.65 L, KF). The suspension was stirred under nitrogen protection and cooled to about 6 °C. Subsequently, phenethyl ether (90 mL, 0.712 mol, 1.01 eq.) was added slowly over 7 minutes, keeping the internal temperature of the reaction below 9 °C. The amount of water in the reaction mixture was 0.003 wt%. The resulting orange solution was diluted with dichloromethane (75 mL) and cooled to -7 °C. Next, a solution of 2-chloro-5-iodobenzoyl chloride (<0.706 mol) in 350 mL of dichloromethane was added dropwise over 13 min, controlling the internal temperature to not exceed the set point. The reaction mixture was warmed slightly and maintained at +5 °C for 2 h. After HPLC analysis confirmed the completion of the reaction, the reaction mixture was quenched by slowly pouring it into 450 mL of pre-cooled (5 °C) aqueous 2N HCl solution. The quenching process was completed in batches over 10 min, ensuring that the internal temperature did not exceed 28°C. The quenched two-phase mixture was stirred at 20 °C for 45 min, and the lower organic phase was separated and washed sequentially with aqueous 1N HCl, saturated aqueous sodium bicarbonate (200 mL each time) and saturated aqueous sodium chloride (200 mL). The washed organic phase was concentrated on a rotary evaporator to give the crude product (2-chloro-5-iodophenyl)(4-ethoxyphenyl)methanone as an off-white solid (268.93 g, HPLC purity 99.0 area %, 220 nm; regional isomer content 1.0 area %, 200 nm; yield 98.5%).
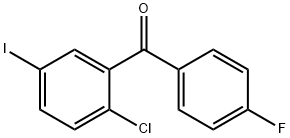
915095-86-2
234 suppliers
$5.00/250mg

141-52-6
502 suppliers
$10.00/5g

1103738-26-6
122 suppliers
$18.00/100mg
Yield: 86.5%
Reaction Conditions:
in N,N-dimethyl-formamide at 20; for 1.5 h;Cooling with ice;Solvent;Temperature;
Steps:
3 Example 3 Preparation of (5-iodo-2-chlorophenyl) (4-ethoxyphenyl) methanone (Compound IIb)
5-iodo-2-chlorophenyl) (4-fluorophenyl) methanone (18.03 g, 50 mmo 1) was added DMF (90 mL), Ice bath slowly add sodium ethoxide (3.748,1. Eq), After adding the reaction at room temperature for 1.5 h, tlc (after the detection reaction is completed, Ice bath slowly add water (180mL), Stirring crystallization lh, Filter, The filter cake was washed with water (200 mL) Beat with ethanol (50 mL) Filtered and dried to give 16.72 g of the pale yellow solid of compound lib, Yield: 86.5% Purity: 99.53%.
References:
Shanghai Pharmaceutical Industry Institute;China Pharmaceutical Industry Zongyuan;Ma Shuai;Zhou Weicheng CN106928040, 2017, A Location in patent:Paragraph 0042-0047

281652-58-2
24 suppliers
inquiry

103-73-1
360 suppliers
$8.00/25g

1103738-26-6
122 suppliers
$18.00/100mg
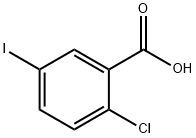
19094-56-5
540 suppliers
$6.00/5g

103-73-1
360 suppliers
$8.00/25g

1103738-26-6
122 suppliers
$18.00/100mg

915095-86-2
234 suppliers
$5.00/250mg
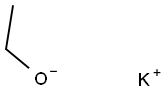
917-58-8
121 suppliers
$43.50/100g

1103738-26-6
122 suppliers
$18.00/100mg

915095-86-2
234 suppliers
$5.00/250mg

64-17-5
758 suppliers
$10.00/50g

1103738-26-6
122 suppliers
$18.00/100mg