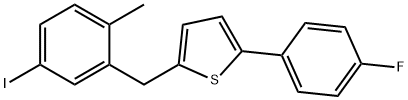
2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene synthesis
- Product Name:2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene
- CAS Number:898566-17-1
- Molecular formula:C18H14FIS
- Molecular Weight:408.27
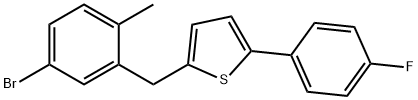
1030825-20-7
![2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene](/CAS/GIF/898566-17-1.gif)
898566-17-1
2-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene (100 g; cf. WO 2005/012326) was dissolved in toluene (300 mL) at room temperature and under nitrogen atmosphere. To this solution, sodium iodide (83 g), cuprous(I) iodide (2.64 g), N,N'-dimethylethylenediamine (2.94 mL) and diethylene glycol dimethyl ether (50 mL) were added sequentially. The reaction mixture was heated to reflux temperature and stirred continuously for 36 hours. Upon completion of the reaction, ethyl acetate (300 mL) was added to the mixture at 40 °C and filtered through a filter pre-coated with activated carbon. The filtrate was washed and concentrated. The resulting residue was suspended in methanol (426 mL) at reflux temperature for 75 minutes. Subsequently, the suspension was cooled to 25 °C and stirring was continued for 1 hour. The precipitate was collected by filtration, washed with methanol and dried under vacuum at 50 °C to afford 2-(5-iodo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene (94.9 g) in white crystalline form. The mass spectrum (APCI) showed a m/z of 409 (M + H); the melting point was 109-110°C. The crystal was found to have the following properties.

1030825-20-7
383 suppliers
$15.00/250mg
![2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene](/CAS/GIF/898566-17-1.gif)
898566-17-1
313 suppliers
$8.00/250mg
Yield:898566-17-1 94.9 g
Reaction Conditions:
with copper(l) iodide;sodium iodide;N,N`-dimethylethylenediamine in diethylene glycol dimethyl ether;toluene for 36 h;Inert atmosphere;Reflux;
Steps:
1 (1) 2-(5-Iodo-2-methylbenzyl)-5-(4-fluorophenyl) thiophene
10098] 2-(5-Bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene (100 g; see WO 2005/012326 pamphlet) was dissolved in toluene (300 mL) at room temperature under N2 atmosphere. Sodium iodide (83 g), copper (I) iodide (2.64 g), N,N’-dimethyl ethylenediamine (2.94 mL) and diglyme (50 mL) was added to the mixture at room temperature. The reaction mixture was heated to reflux temperature and stirred for 36 hours. Ethyl acetate (300 mL) was added to the mixture at 40° C. and the mixture was filtered using activated carbon pre-coated filtet The filtrate was washed and then evaporated. The resulting residue was suspended in methanol (426 mL) at reflux temperature for 75 minutes. The resulting slurry was cooled to 25° C. and stirred for 1 hout The precipitate was filtered and washed with methanol, then dried at 50° C. in vacuo to give 2-(5-iodo-2-methylbenzyl)-5-(4-fluorophenyl) thiophene (94.9 g) as white crystals. mlz (APCI), 409 (M+ H);mp 109-110° C.
References:
MITSUBISHI TANABE PHARMA CORPORATION;SUGIMOTO, Masaaki;KINOSHITA, Hajime;TOKUDA, Takayuki US2016/228375, 2016, A1 Location in patent:Paragraph 0097; 0098
58861-48-6
360 suppliers
$8.00/1g
108440-70-6
10 suppliers
$475.19/250mg
![2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene](/CAS/GIF/898566-17-1.gif)
898566-17-1
313 suppliers
$8.00/250mg

54811-38-0
405 suppliers
$5.00/5g
![2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene](/CAS/GIF/898566-17-1.gif)
898566-17-1
313 suppliers
$8.00/250mg

108440-70-6
10 suppliers
$475.19/250mg
![2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene](/CAS/GIF/898566-17-1.gif)
898566-17-1
313 suppliers
$8.00/250mg

460-00-4
636 suppliers
$6.00/10g
![2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene](/CAS/GIF/898566-17-1.gif)
898566-17-1
313 suppliers
$8.00/250mg