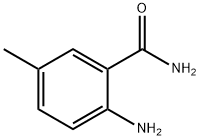
2-AMINO-5-METHYLBENZAMIDE synthesis
- Product Name:2-AMINO-5-METHYLBENZAMIDE
- CAS Number:40545-33-3
- Molecular formula:C8H10N2O
- Molecular Weight:150.18
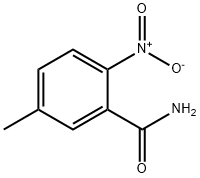
4315-12-2

40545-33-3
Step 2: 5-Methyl-2-nitrobenzamide (15.0 g, 83.3 mmol) was dissolved in methanol (300 mL) and 10% palladium carbon catalyst (2.50 g) was added. The reaction was stirred for 12 hours under hydrogen atmosphere (1 atmosphere) and room temperature. After completion of the reaction, the insoluble material was removed by filtration and the filtrate was concentrated to afford 2-amino-5-methylbenzamide (12.4 g, 99% yield) in white powder form.
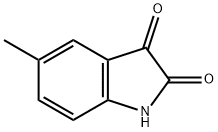
608-05-9
271 suppliers
$10.00/5g

40545-33-3
87 suppliers
$57.50/500 mg
Yield: 85%
Reaction Conditions:
with ammonium hydroxide;dihydrogen peroxide in dimethyl sulfoxide at 20 - 30; for 4 h;Sealed tube;
Steps:
4 Representative procedure for the synthesis of 4 (A)
General procedure: A sealed tube was charged with isatin 1 (1a 147 mg, 1.0 mmol), ammonia hydrate 2 (25%, 421 mg, 3.0 mmol) and H2O2 (30%, 227 mg, 2.0 mmol) at room temperature, and then solvent DMSO (4 mL) was added. The resulting mixture was stirred at 30 °C in a sealed vessel under air after 4 h, then added 50 mL water to the mixture, extracted with CH3COOC2H5 3 times (3 x 50 mL). The extract was washed with 30% NaCl solution (V/V), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (Petroleum ether/Ethyl acetate = 3:1) to yield the desired product 3a as a yellow solid (89% yield).
References:
Wang, Yu-Wei;Zheng, Lei;Jia, Feng-Cheng;Chen, Yun-Feng;Wu, An-Xin [Tetrahedron,2019,vol. 75,# 11,p. 1497 - 1503]

4315-12-2
10 suppliers
$125.00/1 G

40545-33-3
87 suppliers
$57.50/500 mg
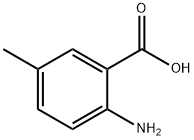
2941-78-8
494 suppliers
$5.00/10g

40545-33-3
87 suppliers
$57.50/500 mg
5925-93-9
142 suppliers
$13.00/250mg

40545-33-3
87 suppliers
$57.50/500 mg

3113-72-2
302 suppliers
$10.00/5g

40545-33-3
87 suppliers
$57.50/500 mg
