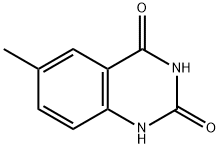
2,4(1H,3H)-Quinazolinedione, 6-methyl- synthesis
- Product Name:2,4(1H,3H)-Quinazolinedione, 6-methyl-
- CAS Number:62484-16-6
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
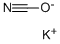
590-28-3
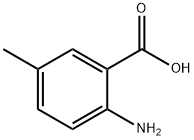
2941-78-8

62484-16-6
Synthesis of 6-methylquinazoline-2,4-dione: 2-amino-5-methylbenzoic acid (0.758 g, 5 mmol) and potassium cyanate (0.673 g, 8.3 mmol) were suspended in water (20 mL) followed by addition of acetic acid (0.5 mL). The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the resulting white solid product was collected by vacuum filtration, washed with water and dried under vacuum to afford 6-methylquinazoline-2,4-dione (0.736 g, 84% yield). The structure of the product was confirmed by 1H NMR (DMSO-d6): δ 9.90 (br s, 1H), 8.27 (d, J = 8.4 Hz, 1H), 7.70 (d, J = 1.8 Hz, 1H), 7.29 (dd, J = 2.4, 8.7 Hz, 1H), 6.50 (br s, 1H), 2.25 (s, 3H).
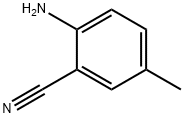
5925-93-9
142 suppliers
$13.00/250mg
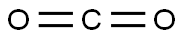
124-38-9
132 suppliers
$175.00/23402

62484-16-6
71 suppliers
$13.00/100mg
Yield: 99%
Reaction Conditions:
with tetrabutyl ammonium fluoride in dimethyl sulfoxide at 110; under 15001.5 Torr; for 24 h;Autoclave;
Steps:
4.4. Synthesis of quinazoline-2,4(1H,3H)-diones 5 from 2-aminobenzonitriles 4 and CO2: general procedure
General procedure: Table 5: To a DMSO-d6 solution (1 mL) of 2-aminobenzonitrile 4a (1 mmol) in a stainless steel autoclave was added a catalyst (0.01 mmol) under an argon atmosphere. The autoclave was sealed, heated at 110°C and then pressurized with CO2 of 2 MPa. The cyclization reaction of 4a proceeded by the magnetic stirring of the resulting mixture at 110°C for 3 h. After the reaction the autoclave was cooled in an ice bath and depressurized. The chemical yield of quinazoline-2,4(1H,3H)-dione 5a was determined by integrating 1HNMR with reference to an internal standard (3,5-dimethoxybenzylalcohol), which was added to the reaction mixture. Table 6: DMSO solution (6 mL) of 4 (6 mmol) was treated for the carboxylative cyclization of 4 with CO2 according to the procedure of Table 5. After the reaction, the autoclave was cooled in an ice bath and depressurized, and the reaction mixture was added to water (60 mL). The precipitation was collected by filtration, washed with water and diethyl ether, and then dried in vacuo at 35°C for 15 h to give the pure product 5.
References:
Fujii, Akira;Matsuo, Hideaki;Choi, Jun-Chul;Fujitani, Tadahiro;Fujita, Ken-ichi [Tetrahedron,2018,vol. 74,# 24,p. 2914 - 2920]
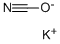
590-28-3
257 suppliers
$10.00/5g

2941-78-8
493 suppliers
$5.00/10g

62484-16-6
71 suppliers
$13.00/100mg

2941-78-8
493 suppliers
$5.00/10g
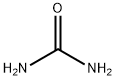
57-13-6
773 suppliers
$5.00/5g

62484-16-6
71 suppliers
$13.00/100mg

5925-93-9
142 suppliers
$13.00/250mg

62484-16-6
71 suppliers
$13.00/100mg
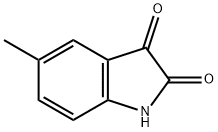
608-05-9
271 suppliers
$10.00/5g

62484-16-6
71 suppliers
$13.00/100mg