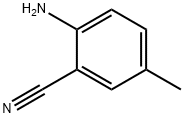
2-Amino-5-Methyl-Benzonitrile synthesis
- Product Name:2-Amino-5-Methyl-Benzonitrile
- CAS Number:5925-93-9
- Molecular formula:C8H8N2
- Molecular Weight:132.16

64113-86-6

5925-93-9
5-Methyl-2-nitrobenzonitrile (1.92 g, 11.84 mmol) was added in batches to a mixed solution of concentrated hydrochloric acid (12 mL) and ethanol (EtOH, 12 mL) with SnCl2 (11.22 g, 59.2 mmol) in stirring. The temperature was controlled at 20-30 °C using an ice bath during the reaction. The reaction mixture was continued to be stirred at room temperature for 1 h. The reaction mixture was then poured into pre-cooled aqueous 6N NaOH solution (ca. 30 mL) and neutralized to pH 7. The product was extracted with ethyl acetate (EtOAc), followed by washing of the organic layer with brine, drying with anhydrous magnesium sulfate (MgSO4), and concentration to give the target product, 2-amino-5-methylbenzonitrile (1.56 g, 99% yield) as a yellowish brown solid. The structure of the product was analyzed by 1H NMR (400 MHz, DMSO-d6) δ 2.21 (s, 3H), 5.79 (bs, 2H), 6.68-6.71 (d, 1H), 7.10-7.13 (dd, 1H), 7.15 (s, 1H); 13C NMR (DMSO-d6) δ 20.13,93.99,116.12, 118.94,125.38,132.32,135.76,150.21; mass spectrum (MS) m/z 133 (MH+) confirmed.

64113-86-6
70 suppliers
inquiry

5925-93-9
141 suppliers
$13.00/250mg
Yield: 99%
Reaction Conditions:
Stage #1:2-nitro-5-methylbenzonitrile with hydrogenchloride;tin(ll) chloride in ethanol;water at 20 - 30; for 1 h;
Stage #2: with sodium hydroxide in ethanol;water; pH=7
Steps:
14.b
5-Methyl-2-nitrobenzonitrile (1.92 g, 11.84 mmol) was added in portions to a stirred solution of SnCl2 (11.22 g, 59.2 mmol) in conc. HCl (12 mL) and EtOH (12 mL). The reaction temperature was maintained at 20-30° C. using an ice bath. The reaction mixture was then stirred at room temperature for 1 h and poured into an ice cold aqueous solution of NaOH (6N, app. 30 mL) to neutralize to pH7. The product was extracted into EtOAc, washed with brine, dried over MgSO4 and concentrated to provide the title product (1.56 g, 99%) as a yellow-brown solid. 1H NMR (400 MHz, DMSO-d6) δ 2.21 (s, 3H), 5.79 (bs, 2H), 6.68-6.71 (d, 1H), 7.10-7.13 (dd, 1H), 7.15 (s, 1H). 13C NMR (DMSO-d6) δ 20.13, 93.99, 116.12, 118.94, 125.38, 132.32, 135.76, 150.21. MS 133 (MH+).
References:
SENOMYX, INC. US2008/306053, 2008, A1 Location in patent:Page/Page column 60

29289-13-2
135 suppliers
$6.00/1g
544-92-3
269 suppliers
$9.00/1g

5925-93-9
141 suppliers
$13.00/250mg
583-68-6
299 suppliers
$10.00/5 g
544-92-3
269 suppliers
$9.00/1g

5925-93-9
141 suppliers
$13.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5925-93-9
141 suppliers
$13.00/250mg
75-16-1
278 suppliers
$12.00/10ml
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5925-93-9
141 suppliers
$13.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry