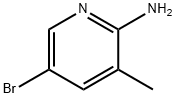
2-Amino-5-bromo-3-methylpyridine synthesis
- Product Name:2-Amino-5-bromo-3-methylpyridine
- CAS Number:3430-21-5
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
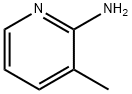
1603-40-3

3430-21-5
General procedure for the synthesis of 2-amino-3-methyl-5-bromopyridine from 2-amino-3-methylpyridine: Bromine (15.4 mL, 0.30 mol) was slowly added dropwise to an anhydrous dichloromethane (1000 mL) solution of 2-amino-3-methylpyridine (32.44 g, 0.30 mol) under stirring at 0-2 °C for 1 hour. The cooling bath was then removed and the yellow suspension was allowed to continue stirring at room temperature for 3 hours and 40 minutes. Upon completion of the reaction, the mixture was alkalized to pH 9 with 2N sodium hydroxide solution (145 mL) and subsequently treated with a mixture of saturated aqueous sodium bicarbonate (100 mL) and saturated aqueous sodium thiosulfate (10 mL). The organic layer was separated, washed with brine, dried over magnesium sulfate and concentrated to give the brown solid product 2-amino-3-methyl-5-bromopyridine (56.83 g, 102% yield), which could be used in the next reaction without further purification.

1603-40-3
485 suppliers
$6.00/25g

3430-21-5
403 suppliers
$5.00/1g
Yield:3430-21-5 100%
Reaction Conditions:
Stage #1:3-methylpyridin-2-ylamine with bromine in dichloromethane at 0 - 20; for 4.66667 h;
Stage #2: with sodium hydroxide in dichloromethane;water; pH=9
Steps:
Bromine (15.4 mL, 0.30 mol) was added dropwise to a stirred and cooled (0-2 [°C)] solution of 2-amino-3-picoline (1; 32.44 g, 0.30 mol) in dry [CH2CI2] (1000 mL) over a period of 1 h. The cooling bath was then removed, and the yellowish suspension was stirred at r. t. for 3 h 40 min. The mixture was basified to pH 9 with 2N [NAOH] (145 mL) followed by a mixture of saturated aqueous [NAHC03] (100 mL) -saturated aqueous Na2S203 (10 mL). The organic layer was separated and washed with brine, dried [MGS04] and concentrated to afford 14 (56.83 g, 102%) as brown solid, which was used in the next step without purification.
References:
EISAI CO., LTD WO2004/13139, 2004, A2 Location in patent:Page 25-26
7463-30-1
69 suppliers
$45.00/10mg

3430-21-5
403 suppliers
$5.00/1g