
5-BROMO-2-METHYLPYRIDIN-3-AMINE synthesis
- Product Name:5-BROMO-2-METHYLPYRIDIN-3-AMINE
- CAS Number:914358-73-9
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
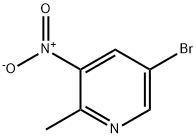
911434-05-4

914358-73-9
The general procedure for the synthesis of 2-methyl-3-amino-5-bromopyridine from 5-bromo-2-methyl-3-nitropyridine was as follows: 5-bromo-2-methyl-3-nitropyridine (13.8 g, 63.9 mmol) was dissolved in industrial methanol (330 mL) at 40 °C, followed by the addition of powdered iron (20 g) in batches to avoid agglomeration. Next, concentrated aqueous hydrochloric acid (5 mL) was added for elution. The resulting dark brown mixture was stirred vigorously under reflux conditions for 2 h. After completion of the reaction, it was cooled to room temperature and filtered through Celite (the filter cake was washed with 1 L of industrial methanol). The filtrate was concentrated under reduced pressure to remove the solvent, and the residue was dissolved in ethyl acetate (200 mL), the organic phase was washed with saturated aqueous sodium bicarbonate (200 mL), and dried with anhydrous magnesium sulfate. Finally, the solvent was removed under reduced pressure to afford the target product 5-bromo-2-methylpyridin-3-amine as an orange solid (10.7 g, 90% yield). The structure of the product was confirmed by 1HNMR (400 MHz, CDCl3, δ): 7.91 (s, 1H), 7.00 (s, 1H), 3.75 (br.s, 2H), 2.25 (s, 3H).

911434-05-4
240 suppliers
$6.00/1g

914358-73-9
201 suppliers
$10.00/1g
Yield:914358-73-9 95%
Reaction Conditions:
with iron;ammonium chloride in methanol;water at 80; for 2 h;
Steps:
4.1.16. 5-bromo-2-methylpyridin-3-amine (16)
2-methyl-3-nitro-5-bromopyridine (15) (13 g, 60 mmol, 1.0 equiv),reduced iron powder (8.4 g, 150 mmol, 2.5 equiv), ammonium chloride(8.1 g, 150 mmol, 2.5 equiv) were added to 100 mL methanol/water (V/V = 4:1) and then transferred to 80 C reflux reaction for 2 h. After thereaction was completed, it was filtered under hot suction, and then thefiltrate was concentrated under reduced pressure to dryness of mostsolvent. Subsequent addition of 100 mL water, suction filtration anddrying it gave a white solid 10.6 g, yield 95%. 1H NMR (400 MHz,CDCl3) δ: 8.29 (d, J = 1.9 Hz, 1H), 7.29 (d, J = 2.1 Hz, 1H), 5.41 (s, 2H),2.28 (s, 3H).
References:
Li, Xiandeng;Yang, Tao;Hu, Mengshi;Yang, Yingxue;Tang, Minghai;Deng, Dexin;Liu, Kongjun;Fu, Suhong;Tan, Yan;Wang, Huan;Chen, Yong;Zhang, Chufeng;Guo, Yong;Peng, Bin;Si, Wenting;Yang, Zhuang;Chen, Lijuan [Bioorganic Chemistry,2022,vol. 121,art. no. 105669]
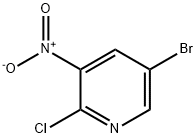
67443-38-3
382 suppliers
$5.00/1g

914358-73-9
201 suppliers
$10.00/1g
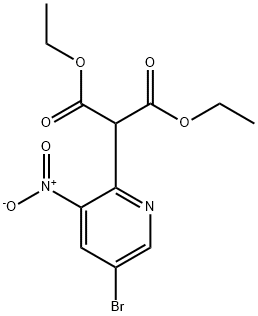
911434-04-3
8 suppliers
inquiry

914358-73-9
201 suppliers
$10.00/1g