
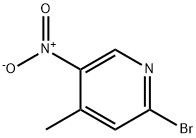
2-BROMO-5-AMINO-4-PICOLINE synthesis
- Product Name:2-BROMO-5-AMINO-4-PICOLINE
- CAS Number:156118-16-0
- Molecular formula:C6H7BrN2
- Molecular Weight:187.04
23056-47-5

156118-16-0
A suspension of 2-bromo-4-methyl-5-nitropyridine (XIV) (200 g, 921 mmol, 1.00 eq.) was formed with NH4Cl (240 g, 4.49 mol, 4.87 eq.) in EtOH (3.50 L) and water (150 mL). The mixture was heated to 50 °C with stirring. Subsequently, Fe (120 g, 2.15 mol, 2.33 eq.) and HCl (10.2 g, 279 mmol, 0.30 eq.) were added to the mixture. The suspension was heated to 80 °C and kept for 3 hours. After completion of the reaction, the mixture was cooled to 25 °C and filtered through a diatomaceous earth plug. The filtrate was concentrated under reduced pressure to give a residue. The residue was dissolved in EtOAc (1L x 3) and washed with brine. The organic layer was dried with sodium sulfate, filtered and concentrated under reduced pressure to give 6-bromo-4-methylpyridin-3-amine (XV) as a brown solid (167.9 g, 898 mmol, 97.4% yield), which could be used for the next reaction without further purification.1H NMR (CDCl3,400 MHz) δ ppm 2.15 (s, 3H), 3.44 (br s 2H), 7.14 (s, 1H), 7.78 (s, 1H); ESIMS m/z 186.8 ([M + H]+).

23056-47-5
270 suppliers
$10.00/1g

156118-16-0
152 suppliers
$11.00/250mg
Yield:156118-16-0 97.4%
Reaction Conditions:
with hydrogenchloride;iron;ammonium chloride in ethanol;water at 50 - 80; for 3 h;
Steps:
A suspension of 2-bromo-4-methyl-5-nitropyridine (XIV) (200 g, 921 mmol, 1.00 eq) and NH4C1 (240 g, 4.49 mol, 4.87 eq) in EtOH (3.50 L) and water (150 mL) was heated with stirring to 50°C. To this mixture was added Fe (120 g, 2.15 mol, 2.33 eq) and HC1 (10.2 g, 279 mmol, 0.30 eq). The suspension was then heated to 80°C for another 3 h. The reaction was cooled to 25°C and filtered through a plug of Celite. The filtrate was concentrated under reduced pressure to yield a residue that was taken up in EtOAc (1 Lx 3) and washed with brine. The organic layer was dried over sodium sulfate, filtered and concentrated under reduced pressure to give 6- bromo-4-methylpyridin-3-amine (XV) as brown solid (167.9 g, 898 mmol, 97.4% yield) which was used for the next step without any purification. ‘H NMR (CDC13, 400 MHz) ppm 2.15 (s, 3H), 3.44 (br s, 2H), 7.14 (s, 1H), 7.78 (s, 1H); ESIMS found for C6H7BrN2 mlz 186.8 (M+H).
References:
SAMUMED, LLC.;KC, Sunil Kumar;WALLACE, David Mark;CAO, Jianguo;CHIRUTA, Chandramouli;HOOD, John WO2017/23975, 2017, A1 Location in patent:Paragraph 0610; 0611