| Identification | More | [Name]
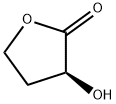
(S)-(-)-alpha-Hydroxy-gamma-butyrolactone | [CAS]
52079-23-9 | [Synonyms]
2,4-DIHYDROXYBUTYRIC ACID LACTONE
(3S)-3-HYDROXYDIHYDROFURAN-2(3H)-ONE
(S)-2-HYDROXYBUTYROLACTONE
(S)-(-)-ALPHA-HYDROXY-GAMMA-BUTYROLACTONE
(S)-(-)-DIHYDRO-3-HYDROXY-2(3H)-FURANONE
(S)-2-Hydroxy-gamma-butyrolactone
2,4-Dihydroxybutyric acid lactone�(S)-2-Hydroxybutyrolactone
(S)-(-)-ALPHA-HYDROXY--BUTYROLACTONE
(S)-(-)-~-Hydroxy-gamma-butyrolactone, 94% | [Molecular Formula]
C4H6O3 | [MDL Number]
MFCD00211245 | [Molecular Weight]
102.09 | [MOL File]
52079-23-9.mol |
| Chemical Properties | Back Directory | [Appearance]
Colourless Liquid | [alpha ]
-68 º (c=1.15 in chloroform) | [Boiling point ]
133 °C10 mm Hg(lit.) | [density ]
1.309 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.467(lit.)
| [Fp ]
>230 °F
| [storage temp. ]
2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Oil | [pka]
13.07±0.20(Predicted) | [color ]
Colourless to Light Yellow | [Specific Gravity]
1.3 | [Optical Rotation]
[α]23/D 68°, c = 1.15 in chloroform | [CAS DataBase Reference]
52079-23-9(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
Colourless Liquid | [Uses]
(3S)-3-Hydroxydihydrofuran-2(3H)-one (cas# 52079-23-9) is a compound useful in organic synthesis. | [Uses]
Used to prepare the polyether antibiotic monensin, functionalized D-ring side chains of vitamin D analogs, and pesticides. | [Synthesis]
Example 1: Synthesis of (S)-3-hydroxydihydrofuran-2(3H)-one
1. 10.0 g of L-malic acid was dissolved in 45 mL of trifluoroacetic anhydride and the reaction was stirred at 25 °C for 2 hours.
2. After completion of the reaction, the solvent was removed by distillation under reduced pressure to obtain a residue.
3. 7 mL of methanol was added to the residue and stirring was continued for 12 h. 4.
4. The reaction mixture was again concentrated by distillation under reduced pressure. 5.
5. The resulting residue was dissolved in 150 mL of anhydrous tetrahydrofuran, and the solution was cooled to 0 °C.
6. 150 mL of borane-tetrahydrofuran complex was slowly added at 0 °C and stirring was continued for 2.5 hours while maintaining temperature.
7. Upon completion of the reaction, 150 mL of methanol was added and stirred at room temperature for 1 hour.
8. The reaction mixture was concentrated by distillation under reduced pressure.
9. The crude product was dissolved in 80 mL of toluene, 5.0 g of activated acidic Dowex resin was added and refluxed for 1 hour.
10. After completion of the reaction, the Dowex resin was removed by filtration and the filtrate was concentrated by distillation under reduced pressure.
11. 7.61 g of crude product (99.9% yield) was obtained, which was directly used in the subsequent reaction without further purification. | [References]
[1] Patent: US7001916, 2006, B1. Location in patent: Page/Page column 70
[2] European Journal of Organic Chemistry, 2009, # 18, p. 2987 - 2997 |
|
|