| Identification | More | [Name]
5-Bromo-2-nitropyridine | [CAS]
39856-50-3 | [Synonyms]
2-NITRO-5-BROMOPYRIDINE
3-BROMO-6-NITROPYRIDINE
5-BROMO-2-NITROPYRIDINE
AKOS BBS-00001330
2-NITRO-5-BROMO PYRIDINE(5-BROMO-2-NITROPYRIDINE)
5-BROMO-2-NITROPYRIDINE, 98+%
3-Bromo-6-nitropyridine (5-Bromo-2-nitropyridine)
5-Bromo-2-notropyridine | [EINECS(EC#)]
609-748-8 | [Molecular Formula]
C5H3BrN2O2 | [MDL Number]
MFCD00160411 | [Molecular Weight]
202.99 | [MOL File]
39856-50-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
148-150 °C (lit.) | [Boiling point ]
292.3±20.0 °C(Predicted) | [density ]
1.833±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
-4.95±0.22(Predicted) | [color ]
White to yellow to beige powder or crystal or chunks | [BRN ]
120878 | [InChI]
InChI=1S/C5H3BrN2O2/c6-4-1-2-5(7-3-4)8(9)10/h1-3H | [InChIKey]
ATXXLNCPVSUCNK-UHFFFAOYSA-N | [SMILES]
C1([N+]([O-])=O)=NC=C(Br)C=C1 | [CAS DataBase Reference]
39856-50-3(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [PackingGroup ]
III | [HS Code ]
29333990 |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless solid | [Uses]
5-Bromo-2-nitropyridine is employed as a reagent in the synthesis of novel benzinidazoles, potent inhibitors of TIE-2 and VEGFR-2 Tyrosine (T899975) kinase receptors. | [Preparation]
5-Bromo-2-nitropyridine was prepared from the corresponding amine via hydrogen peroxide oxidation in large scale production. N-Bromosuccinimide (NBS) bromination of 2-aminopyridine in acetonitrile at 0-5 °C delivered 5-bromo-2- aminopyridine with good regioselectivity (>20:1). The brominated amino-pyridine was then reacted with the oxidant mixture, yielding 5-Bromo-2-nitropyridine after dilution with water and isopropanol[1].
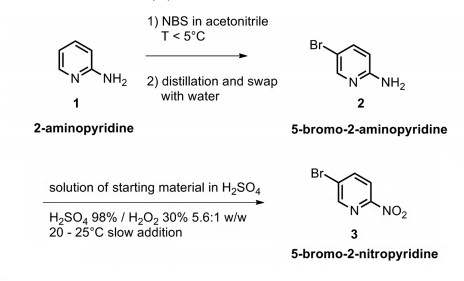 | [Synthesis Reference(s)]
[1] Agosti, Alessandro, et al. "Handling Hydrogen Peroxide Oxidations on a Large Scale: Synthesis of 5-Bromo-2-nitropyridine." Organic Process Research & Development 21.3(2017):451-459. | [Synthesis]
General steps for the synthesis of 5-bromo-2-nitropyridine from 2-amino-5-bromopyridine: Add 500L of glacial acetic acid and 150L of acetic acid in a 1000L autoclave, turn on the stirring and circulate the cooling water. When the mixture is homogeneous, slowly add 120 kg of 2-amino-5-bromopyridine and control the temperature at 20℃. After stirring for 30 minutes, 300 kg of peroxyacetic acid was slowly added dropwise. When the dropwise addition to the remaining about 50kg, observed that the temperature began to rise slowly, at this time to suspend the dropwise addition. After the temperature stabilized and stopped rising, the remaining peroxyacetic acid was added dropwise, and the temperature was kept at 30℃ throughout the process. After the dropwise addition was completed, the temperature was raised to 40°C and the reaction was kept at a constant temperature for 20 hours. After the reaction was completed, distillation was carried out under reduced pressure, and the distillation was stopped after about 600 L of acetic acid was evaporated. The remaining liquid was cooled to 25 °C, diluted by adding 500 L of water, and the pH was adjusted with 40% sodium hydroxide solution to 8. Subsequently, the mixture was cooled to -20 °C, filtered and dried to give 125 kg of crude product in 89.3% yield. After recrystallization, 120.5 kg of pure product was obtained in 86.1% yield. | [References]
[1] Patent: CN106187867, 2016, A. Location in patent: Paragraph 0035; 0044; 0045
[2] Bioorganic and Medicinal Chemistry, 1999, vol. 7, # 3, p. 467 - 479
[3] Patent: CN108558745, 2018, A. Location in patent: Paragraph 0023; 0024; 0025; 0026
[4] Patent: WO2008/62182, 2008, A1. Location in patent: Page/Page column 113
[5] Journal of Organic Chemistry, 2008, vol. 73, # 23, p. 9326 - 9333 |
|
|