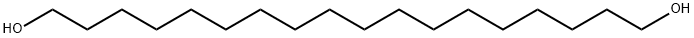[Synthesis]
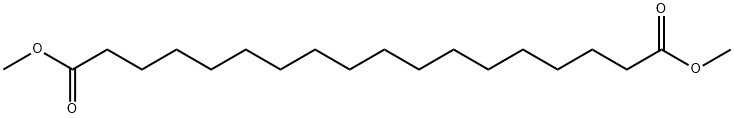
The general procedure for the synthesis of 1,18-octadecanediol from dimethyl octadecanedioate was as follows: in a 1000 mL three-necked flask, dimethyl octadecanedioate (14.8 g, 43.1 mmol, 1.0 eq.) was dissolved in 700 mL of anhydrous tetrahydrofuran (THF) and the reaction system cooled down to 0 °C. Subsequently, lithium aluminum hydride (LiAlH4, 4.1 g, 107.8 mmol, 2.5 eq.) was slowly added. The resulting reaction mixture was stirred at room temperature for 1 hour and subsequently heated to reflux for 6 hours. Upon completion of the reaction, the mixture was cooled again to 0 °C and the reaction was quenched by slow addition of a pre-cooled aqueous sulfuric acid solution (4 equivalents of sulfuric acid versus 12 equivalents of LiAlH4 in water) in THF. The precipitate was removed by filtration and the solid was washed with THF (3 x 400 mL). The filtrate was concentrated under reduced pressure to give the white solid product 1,18-octadecanediol (12.3 g, 98% yield).
1H NMR (400 MHz, THF-d8, TMS): δ (ppm) = 3.45 (dd, J = 11.35, 6.15 Hz, 4H, HO-CH2-CH2-), 2.49 (s, 2H, HO-CH2-), 1.46 (m, 4H, HO-CH2-CH2-), 1.39-1.25 (m, 24H, - CH2-(CH2)12-CH2-).
13C NMR (100 MHz, THF-d8, TMS): δ (ppm) = 62.7 (HO-CH2-), 34.3 (HO-CH2-CH2-), 30.9-30.7 (-CH2-(CH2)12-CH2-), 27.1 (HO-(CH2)2-CH2-).
IR (cm-1): 3414, 3355, 2917, 2846, 1468, 1352, 1334, 1059, 1046, 1015, 979, 723.
MS (EI): m/z (%) Calculated C18H38O2Na: 309.2770; measured: 309.2770. |