| Identification | More | [Name]
GW9662 | [CAS]
22978-25-2 | [Synonyms]
CS-762
GW9662
GW9662, >=98%
GW9662; GW 9662
GW9662 USP/EP/BP
TIMTEC-BB SBB006523
2-CHLORO-5-NITROBENZANILIDE
2-Chloro-5-nitrobenzanilide 97%
2-CHLORO-5-NITRO-N-PHENYLBENZAMIDE
GW9662;GW 9662;TIMTEC-BB SBB006523
GW9662 - CAS 22978-25-2 - Calbiochem
2-Chloro-5-nitro-N-4-phenylbenzamide
BenzaMide,2-chloro-5-nitro-N-phenyl-
N-[(2-chloro-5-nitrophenyl)-phenylmethylidene]hydroxylamine | [EINECS(EC#)]
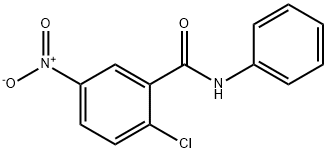
636-590-7 | [Molecular Formula]
C13H9ClN2O3 | [MDL Number]
MFCD01215270 | [Molecular Weight]
276.68 | [MOL File]
22978-25-2.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-White Solid | [Melting point ]
158-159 °C (lit.) | [Boiling point ]
360.9±32.0 °C(Predicted) | [density ]
1.440±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
DMSO: 26 mg/mL, soluble
| [form ]
solid
| [pka]
11.77±0.70(Predicted) | [color ]
white
| [Usage]
A cell-permeable, selective and irreversible PPAR antagonist (IC50 = 3.3 nM, 32 nM, and 2 for PPAR, PPARa, and PPARd, respectively). Reported to covalently modify a cysteine residue in the binding site of PPAR. At a concentration of 10 , also | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. | [InChIKey]
DNTSIBUQMRRYIU-UHFFFAOYSA-N | [CAS DataBase Reference]
22978-25-2(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36:Irritating to the eyes.
R43:May cause sensitization by skin contact. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S24/25:Avoid contact with skin and eyes .
S22:Do not breathe dust . | [WGK Germany ]
3
| [HS Code ]
29242990 |
| Hazard Information | Back Directory | [Description]
GW-9662 (22978-25-2) is a selective PPARγ antagonist (IC50 = 3.3, 32 and 2000 nM for PPARγ, PPARα and PPARδ respectively).1 Blocks the inhibition of osteoclast formation induced by IL-4 (1-2 μM).2 Displays anticancer activity inhibits growth of human mammary tumor cell lines.3 GW-9662 is a useful tool for dissecting the involvement of PPARγ in cellular physiology.4,5 | [Chemical Properties]
Off-White Solid | [Uses]
A cell-permeable, selective and irreversible PPAR antagonist (IC50 = 3.3 nM, 32 nM, and 2 for PPAR, PPARa, and PPARd, respectively). Reported to covalently modify a cysteine residue in the binding site of PPAR. At a concentration of 10 , also | [Definition]
ChEBI: GW 9662 is a member of benzamides. | [Biological Activity]
Selective PPAR γ antagonist (IC 50 values are 3.3, 32 and 2000 nM for PPAR γ , PPAR α and PPAR δ respectively). Blocks the inhibition of osteoclast formation induced by IL-4 in the low micromolar range (1-2 μ M), therefore is more potent than BADGE (2,2-Bis[4-(2,3-epoxypropoxy)phenyl]propane ). Anticancer, inhibits growth of human mammary tumor cell lines. | [Biochem/physiol Actions]
GW9662 (2-chloro-5-nitrobenzanilide) binds to the ligand binding site of the peroxisome proliferator activated receptor γ (PPARγ) and results in the inhibition of adipocyte differentiation. It favors cell growth suppression in breast cancer cell lines even in the presence of PPARγ agonist rosiglitazone. It stimulates M2c macrophages differentiation and triggers growth arrest-specific 6 (Gas6) expression. GW9662 co treatment with other PPARγ ligands elicits antiproliferative effects on the glioblastoma stem cells and could be a potent therapeutic agent. | [Synthesis]
To a stirred solution of 2-chloro-5-nitrobenzoyl chloride (5.03 g, 22.9 mmol) and triethylamine (3.51 mL, 25.1 mmol) in dichloromethane (CH2Cl2) maintained at 0°C in a nitrogen atmosphere was slowly added aniline (2.19 mL, 24.0 mmol) dropwise. The reaction mixture was continued to be stirred at 0 °C for 5 min, then brought to room temperature and stirred for 15 min. Upon completion of the reaction, the solution was diluted with ethyl acetate (EtOAc, 300 mL) and washed sequentially with 1.0 M HCl, water, 1.0 M NaHCO3 and saturated saline (100 mL each). The organic phase was dried over anhydrous magnesium sulfate (MgSO4) and concentrated by rotary evaporation to give a light yellow solid (5.32 g). The solid was purified by recrystallization from ethyl acetate to give a white solid 2-chloro-5-nitro-N-phenylbenzamide (3.34 g, 53% yield) with a melting point of 155-156 °C. The product was confirmed by 1H NMR (CDCl3, 400 MHz): δ 8.63 (d, 1H, J=2.7 Hz), 8.28 (dd, 1H, J=2.7, 8.9 Hz), 7.81 (br s, 1H), 7.68-7.63 (m, 3H), 7.42 (t, 2H, J=7.9 Hz), 7.23 (t, 1H, J= 7.5 Hz). The mass spectrum (ES-) showed m/z 275.1 ([M-H]-). Elemental analysis (C13H9ClN2O3) calculated values: C, 56.43; H, 3.28; N, 10.13; measured values: C, 56.33; H, 3.30; N, 10.03. | [storage]
Store at -20°C | [References]
1) Leesnitzer et al. (2002), Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662; Biochemistry, 41 6640
2) Bendixen et al. (2001), IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1; Proc. Natl. Acad. Sci. USA, 98 2443
3) Seargent et al. (2004), GW9662, a potent antagonist of PPARgamma inhibits growth of breast cancer tumour cells and promotes the anticancer effects of the PPARgamma agonist rosiglitazone, independently of PPARgamma activation; Br. J. Pharmacol., 143 933
4) Cheng et al. (2014), β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway; Pharmacology, 94 1
5) Liu et al. (2014), Curcumin protects neurons against oxygen-glucose deprivation/reoxygenation-induced injury through activation of peroxisome proliferator-activated-γ function; J. Neuro. Sci. Res., 92 1549 |
|
|