| Identification | More | [Name]
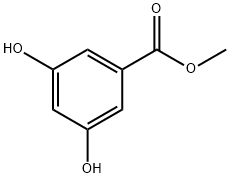
Methyl 3,5-dihydroxybenzoate | [CAS]
2150-44-9 | [Synonyms]
3,5-DIHYDROXYBENZOIC ACID METHYL ESTER
AKOS 227-38
ALPHA-RESORCYLIC ACID METHYL ESTER
METHYL 3,5-DIHYDROXYBENZOATE
METHYL-A-RESORCYLATE
RARECHEM AL BF 0252
3,5-dihydroxy-benzoicacimethylester
Resorcinol-5-carboxylicacidmethylester
Benzoic acid, 3,5-dihydroxy-, methyl ester
Methyl alpha-resorcylate
3,5-Dihydroxybenzoic acid methyl
α-Resorcylic acid methyl | [EINECS(EC#)]
218-426-2 | [Molecular Formula]
C8H8O4 | [MDL Number]
MFCD00002289 | [Molecular Weight]
168.15 | [MOL File]
2150-44-9.mol |
| Chemical Properties | Back Directory | [Appearance]
slightly yellow to beige fine crystalline powder | [Melting point ]
167-170 °C (lit.) | [Boiling point ]
257.07°C (rough estimate) | [density ]
1.3037 (rough estimate) | [refractive index ]
1.5090 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Acetone (Slightly), Methanol (Slightly) | [form ]
Fine Crystalline Powder | [pka]
8.63±0.10(Predicted) | [color ]
Slightly yellow to beige | [Water Solubility ]
Slightly soluble in water. | [BRN ]
2091651 | [InChI]
InChI=1S/C8H8O4/c1-12-8(11)5-2-6(9)4-7(10)3-5/h2-4,9-10H,1H3 | [InChIKey]
RNVFYQUEEMZKLR-UHFFFAOYSA-N | [SMILES]
C(OC)(=O)C1=CC(O)=CC(O)=C1 | [CAS DataBase Reference]
2150-44-9(CAS DataBase Reference) | [EPA Substance Registry System]
2150-44-9(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29182900 |
| Hazard Information | Back Directory | [Chemical Properties]
slightly yellow to beige fine crystalline powder | [Uses]
The methyl ester of 3,5-Dihydroxybenzoic Acid (D451700) with potential antifeedant activity for pine weevil, Hylobius abietis. | [General Description]
Ribonucleotide reductase inhibiton and antiumor activity of methyl 3,5-dihydroxybenzoate has been studied. | [Synthesis]
Methyl 3,5-dihydroxybenzoate was synthesized from methanol and 3,5-dihydroxybenzoic acid using the method described by Brouwer et al. The method was adapted for the metathesis synthesis of amino acid-based dendritic polymers, aiming at obtaining dendritic macromolecules with alkyne groups on the surface for 1,3-dipolar cycloaddition ("click") reactions with amino acid/peptide-derived azides. This was done as follows: first, 3,5-dihydroxymethylbenzoate (21.4 g, 130 mmol) was dissolved in anhydrous DMF (250 mL), followed by the addition of anhydrous K2CO3 (45 g, 330 mmol, 2.5 eq.). A toluene solution of propargyl bromide (35 mL, 314 mmol, 2.5 eq.) was added slowly and dropwise to this suspension. The reaction mixture was stirred continuously for 48 hours at room temperature. Upon completion of the reaction, DMF was removed by evaporation and the residue was redissolved in EtOAc (400 mL). The organic phase was washed sequentially with H2O (3 × 100 mL), KHSO4 (3 × 100 mL) and brine (3 × 100 mL), after which it was dried with Na2SO4 and evaporated under vacuum. The residue was recrystallized by EtOAc/hexane to afford the target product 1 as off-white crystals in 81% yield (25.2 g). The product was characterized by thin layer chromatography (TLC) and nuclear magnetic resonance (NMR): Rf (EtOAc/hexane 4:1 v/v): 0.76; Rf (DMC/MeOH 98:2 v/v): 0.87; Rf (CHCl3/MeOH/AcOH 95:20:3 v/v): 0.83; 1H-NMR (CDCl3) δ 2.55 (t, J=2.47Hz, 2H), 3.91 (s, 3H), 4.72 (d, J=2.47Hz, 4H), 6.81 (t, J=2.20Hz, 1H), 7.29 (d, J=2.20Hz, 2H); 13C-NMR (CDCl3) δ 52.4, 56.0, 76.0, 77.9, 107.5, 108.8, 132.0, 157.8, 158.4; elemental analysis results: C14H12O4, calculated value C 68.83, H 4.95, measured value C 68.76, H 4.95. | [References]
[1] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1981, p. 2825 - 2829
[2] Chemistry Letters, 1999, # 11, p. 1193 - 1194
[3] Chemical Communications, 2005, # 36, p. 4581 - 4583
[4] Patent: EP1733742, 2006, A1. Location in patent: Page/Page column 5-6; 12
[5] Journal of the American Chemical Society, 2007, vol. 129, # 10, p. 2774 - 2776 |
|
|