| Identification | More | [Name]
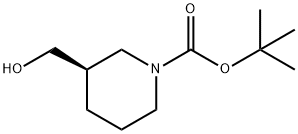
(R)-1-Boc-3-(hyroxymethyl)piperidine | [CAS]
140695-85-8 | [Synonyms]
3-(R)-N-BOC-PIPERIDINE METHANOL
BOC-R-PIP-3MEOH
N-BOC-(3R)-PIP(3-CH2OH)
(R)-1-BOC-3-(HYDROXYMETHYL)PIPERIDINE
(R)-1-N-BOC-3-(HYDROXYMETHYL)PIPERIDINE
(R)-3-HYDROXYMETHYL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
(R)-N-ALPHA-T-BUTYLOXYCARBONYL-3-PIPERIDINEMETHANOL
(R)-N-ALPHA-TERT-BUTYLOXYCARBONYL-3-PIPERIDINEMETHANOL
(R)-N-BOC-3-HYDROXYMETHYL PIPERIDINE
(R)-TERT-BUTYL 3-(HYDROXYMETHYL)PIPERIDINE-1-CARBOXYLATE
TERT-BUTYL (R)-3-HYDROXYMETHYLPIPERIDINE-1-CARBOXYLATE
(R)-N-Boc-3-piperidinemethanol
(R)-1-BOC-3-(HYROXYMETHYL)PIPERIDINE
(R)-N-t-Butyloxycarbonyl-3-(hydroxymethyl)piperidine, (R)-Boc-Nip-ol
1-Piperidinecarboxylic acid, 3-(hydroxymethyl)-, 1,1-dimethylethyl ester, (3R)-
(R)-3-(Hydroxymethyl)piperidine, N-BOC protected
(R)-N-Boc-3-(hyroxymethyl)piperidine
(R)-N-tert-butoxycarbonyl-3-piperidinemethanol | [Molecular Formula]
C11H21NO3 | [MDL Number]
MFCD02683202 | [Molecular Weight]
215.29 | [MOL File]
140695-85-8.mol |
| Chemical Properties | Back Directory | [Melting point ]
77-81 °C | [Boiling point ]
308.0±15.0 °C(Predicted) | [density ]
1.059±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [form ]
Powder | [pka]
14.93±0.10(Predicted) | [color ]
White to yellow | [Water Solubility ]
Slightly soluble in water. | [InChI]
InChI=1S/C11H21NO3/c1-11(2,3)15-10(14)12-6-4-5-9(7-12)8-13/h9,13H,4-8H2,1-3H3/t9-/m1/s1 | [InChIKey]
OJCLHERKFHHUTB-SECBINFHSA-N | [SMILES]
N1(C(OC(C)(C)C)=O)CCC[C@@H](CO)C1 | [CAS DataBase Reference]
140695-85-8(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
(R)-(-)-1-Boc-3-(hydroxymethyl)piperidine is used as pharmaceutical intermediate. | [Synthesis]
The general procedure for the synthesis of tert-butyl 3-(hydroxymethyl)piperidine-1-carboxylate from (R)-1-(tert-butoxycarbonyl)piperidine-3-carboxylic acid is as follows:
Example 58: Synthesis of N-(2-methylpropyl)-N-(3,5-dichlorophenyl)methyl-(3R)-piperidin-3-ylmethylamine 1.5L-tartrate:
(i) Borane-tetrahydrofuran complex (1 M solution of THF, 65.4 mL, 65.4 mmol, 3 eq.) was slowly added dropwise to a solution of (R)-(-)-N-Boc-piperidinic acid (5 g, 21.8 mmol, 1 eq.) in THF (50 mL) at room temperature. The reaction mixture was stirred at room temperature for 16 hours. Subsequently, the solution was cooled to 0 °C and hydrolyzed by careful addition of 2N aqueous sodium hydroxide solution (250 mL). After hydrolysis, the mixture was heated and kept for 48 hours. After completion of the reaction, the mixture was again cooled to 0 °C and extracted with ether (3 x 100 mL). The organic layers were combined, washed with brine (100 mL), dried over magnesium sulfate, filtered and concentrated in vacuum. The residue was purified by fast chromatography using an isohexane solution of 50-70% ethyl acetate as eluent to afford tert-butyl (3R)-3-(hydroxymethyl)piperidine-1-carboxylate (4.56 g, 98% yield).
1H NMR (300 MHz, CDCl3) δ: 4.0-2.7 (6H, br m), 2.17-1.19 (5H, br m), 1.46 (9H, s). | [References]
[1] Patent: WO2005/118531, 2005, A1. Location in patent: Page/Page column 74
[2] Patent: WO2006/12308, 2006, A1. Location in patent: Page/Page column 68
[3] Patent: WO2017/103611, 2017, A1. Location in patent: Paragraph 00298 |
|
|