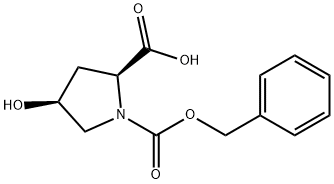
Z-CIS-HYP-OH synthesis
- Product Name:Z-CIS-HYP-OH
- CAS Number:13504-86-4
- Molecular formula:C13H15NO5
- Molecular Weight:265.26
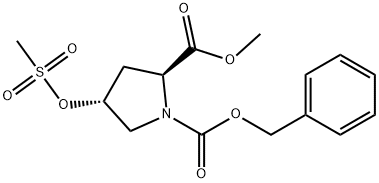
117811-78-6

13504-86-4
General procedure for the synthesis of (2S,4S)-N-Cbz-4-hydroxypyrrolidine-2-carboxylic acid from (2S,4R)-Cbz-4-methanesulfonyloxyproline methyl ester: firstly, 708 g of water and 337 g of 48% sodium hydroxide solution were added to 928 g of the crude product obtained by reference to Example 3. The temperature of the mixture was raised to 80°C and the reaction was ripened at this temperature for 3 hours. Upon completion of the maturation, the temperature was kept constant and 146 g of solvent was distilled under reduced pressure until the pressure was reduced to 13 kPa. Subsequently, the mixture was cooled to 20°C, 180 g of 48% sodium hydroxide solution was added and aged at 25°C for 12 hours. The amount of cis-Benzyloxycarbonyl-4-hydroxy-L-proline in the reaction solution was determined by analysis to be 2.19 mol (81.0% yield based on trans-4-hydroxy-L-proline). Next, 35 g of toluene was added to the hydrolysis reaction solution and the mixture was concentrated under reduced pressure at 7 kPa and 40 °C to distill 332 g of solvent. Then, 161 g of concentrated sulfuric acid was added dropwise to the concentrated solution and the pH was adjusted to 1.3. After acidic adjustment, 710 g of tetrahydrofuran and 354 g of water were added for layered separation. To the separated water layer, 356 g of tetrahydrofuran was added and the separation was performed again. All the separated tetrahydrofuran layers were combined and concentrated at 40 °C under reduced pressure at 26 kPa, resulting in 896 g of concentrate containing 2.12 mol of cis-Benzyloxycarbonyl-4-hydroxy-L-proline (90.2% yield) with a cis/trans ratio of 98.3/1.7.

117811-78-6
17 suppliers
$394.31/1g

13504-86-4
85 suppliers
$18.00/100mg
Yield: 90.2%
Reaction Conditions:
with water;sodium hydroxide at 80; for 3 h;
Steps:
4 Reference Example 4
708 g of water and 337 g of 48% sodium hydroxide were added to 928 g of the crude product obtained in Reference Example 3, and the temperature was raised to 80 ° C., followed by reaction ripening for 3 hours. After completion of the aging, 146 g was distilled out under reduced pressure to 13 kPa under the same temperature condition. After concentration, the temperature was cooled to 20 ° C., 180 g of 48% caustic soda was added, and aging was carried out at 25 ° C. for 12 hours. The content of cis-benzyloxycarbonyl-4-hydroxy-L-proline in the reaction solution was 2.19 mol (the yield based on trans-4-hydroxy-L-proline was 81.0%). 35 g of toluene was added to the hydrolysis reaction solution, and the mixture was concentrated under reduced pressure at 7 kPa at 40 ° C. to distill off 332 g. To the concentrated solution, 161 g of concentrated sulfuric acid was added dropwise to adjust the pH to 1.3. After adjusting to acidity, 710 g of tetrahydrofuran and 354 g of water were added and the layers were separated. To the separated aqueous layer side, 356 g of tetrahydrofuran was added and the layers were separated. The separated tetrahydrofuran layer side was mixed and concentrated at 40 ° C. under 26 kPa reduced pressure to obtain 896 g of a concentrate (cis-benzyloxycarbonyl-4-hydroxy-L-proline 2.12 mol (yield 90. 2%), cis / trans = 98.3 / 1.7)
References:
TORAY FINE CHEMICALS COMPANY LIMITED;NISHIMURA, TOMOAKI;MORIMOTO, MASAO JP5703654, 2015, B2 Location in patent:Paragraph 0054; 0055
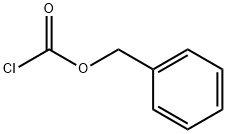
501-53-1
417 suppliers
$10.00/1g
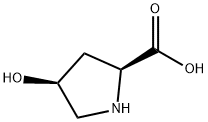
618-27-9
313 suppliers
$9.00/250mg

13504-86-4
85 suppliers
$18.00/100mg
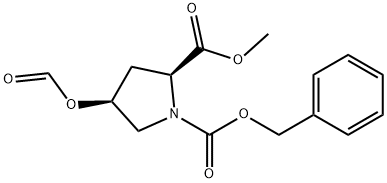
170159-62-3
1 suppliers
inquiry

13504-86-4
85 suppliers
$18.00/100mg

13504-85-3
288 suppliers
$5.00/1g

13504-86-4
85 suppliers
$18.00/100mg
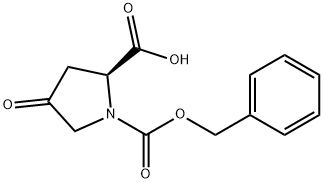
64187-47-9
94 suppliers
$35.10/250mg

13504-86-4
85 suppliers
$18.00/100mg