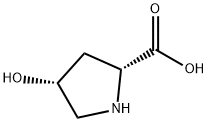
cis-4-Hydroxy-D-proline synthesis
- Product Name:cis-4-Hydroxy-D-proline
- CAS Number:2584-71-6
- Molecular formula:C5H9NO3
- Molecular Weight:131.13
51-35-4

2584-71-6
General procedure for the synthesis of cis-4-hydroxy-D-proline using L-hydroxyproline as starting material: a synthetic route has been successfully developed for the other two stereoisomers in the pro4 monomer class. A controlled differential isomerization strategy [34] was employed to convert 30 g of trans-4-hydroxy-(L)-proline 5 into its diastereoisomer cis-4-hydroxy-(D)-proline 14 in 57% isolated yield. Further processing of the intermediate by the synthetic method described in Scheme 1 allowed the synthesis of two additional enantiomers of the pro4 structural unit class 3, pro4(2R,4R) and pro4(2R,4S) (see Scheme 3 for details).
132666-67-2
14 suppliers
inquiry

2584-71-6
339 suppliers
$6.00/1g
Yield:2584-71-6 253 mg
Reaction Conditions:
with water;sodium hydroxide in ethanol; for 2 h;
Steps:
(2R, 4R)-4-hydroxyproline(1):
A solution of 5a (600 mg, 2.41 mol) in ethanol (9 mL)was subjected to hydrogenation under 50 psi hydrogen pressure in presence of10% Pd(OH)2 (150 mg) for 3 h. The catalyst was filtered off and thesolution evaporated to dryness. Crude charged with 6 mL, 1M NaOH solution andstirred for 2 h. The reaction mass acidified with acetic acid to pH ~ 5 andpurified with Indion 225 H resin, product eluted in 5% aqueous ammonia. Productfraction evaporated to get solid, which was further purified with ethanol (5 mL)to afford 253 mg of with 80% yield.MP = 248-251 °C(decomposition), [α]D25 = +55.87° (c 1.0, H2O),lit.3, [α]D25 = +58.6° (c 0.65, H2O).1H NMR (400 MHz, D2O): δ ppm2.18-2.23 (m, 1H), 2.42-2.49 (m, 1H), 3.32 (dd, J = 3.9 Hz, 1H), 3.41 (d, J = 12.3 Hz, 1H), 4.16 (dd, J = 3.9 Hz, 1H), 4.53 (m, 1H).; 13CNMR (100 MHz, D2O): δ ppm 37.06,82.85, 59.59, 69.05, 174.43.; IR: 3213, 1633, 1564, 1433, 1383.; HRMS (ESI):Calcd for C5H8O3 (M-H)+ 130.0504, Found130.0501. MP = 248-251 °C (decomposition), [α]D25= +55.87° (c 1.0, H2O), lit.3, [α]D25= +58.6° (c 0.65, H2O).
References:
Gajare, Vikas S.;Khobare, Sandip R.;Malavika;Rajana, Nagaraju;Venkateswara Rao;Syam Kumar [Tetrahedron Letters,2015,vol. 56,# 24,p. 3743 - 3746] Location in patent:supporting information

51-35-4
887 suppliers
$6.00/5g

2584-71-6
339 suppliers
$6.00/1g
![2-Oxa-5-azabicyclo[2.2.1]heptan-3-one, 5-acetyl-, (1R,4R)- (9CI)](/CAS/GIF/444313-67-1.gif)
444313-67-1
5 suppliers
inquiry

2584-71-6
339 suppliers
$6.00/1g
135042-12-5
197 suppliers
$6.00/1g

2584-71-6
339 suppliers
$6.00/1g
![2-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-4,5-dihydroxy-, ethyl ester, (2Z,4R)-](/CAS/20210305/GIF/502442-54-8.gif)
502442-54-8
0 suppliers
inquiry

2584-71-6
339 suppliers
$6.00/1g