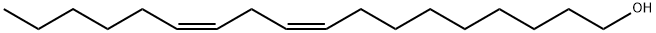
CIS,CIS-9,12-OCTADECADIENOL synthesis
- Product Name:CIS,CIS-9,12-OCTADECADIENOL
- CAS Number:506-43-4
- Molecular formula:C18H34O
- Molecular Weight:266.46
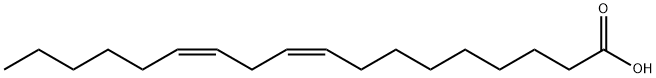
60-33-3
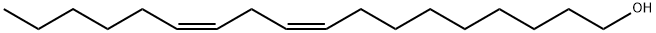
506-43-4
The general procedure for the synthesis of (9Z,12Z)-octadeca-9,12-dien-1-ol from linoleic acid was as follows: lithium aluminum hydride (2.73 g, 72 mmol) was suspended in tetrahydrofuran (THF, 190 mL) cooled to 4 °C. Under stirring, linoleic acid (10 g, 36 mmol) was slowly added dropwise to the above suspension, and stirring was continued for 10 min after completion of the dropwise addition. Subsequently, the reaction mixture was placed in an oil bath and heated to reflux overnight. After completion of the reaction, it was cooled to room temperature and the reaction was quenched by slow addition of 1 mol/L aqueous sodium hydroxide solution (100 mL). The reaction mixture was diluted with ethyl acetate (100 mL) and filtered to remove insoluble material. The filtrate was washed with saturated aqueous sodium bicarbonate, and the organic layer was separated and dried over anhydrous sodium sulfate. After filtration, the solvent was removed by rotary evaporator to obtain the crude product. The crude product was purified by silica gel column chromatography using a continuous gradient of hexane with ethyl acetate as eluent to afford (9Z,12Z)-octadeca-9,12-dien-1-ol (8.68 g, 32.6 mmol) as a colorless oil in 91% yield. The structure of the product was confirmed by proton nuclear magnetic resonance (1H NMR, 500 MHz) with the following chemical shifts: δ= 0.88 (t, 3H), 1.25-1.36 (m, 16H), 1.53-1.58 (m, 2H), 2.02-2.06 (m, 4H), 2.76 (t, 2H), 3.62 (t, 2H), 5.29-5.40 (m, 4H).

60-33-3
609 suppliers
$9.00/5g
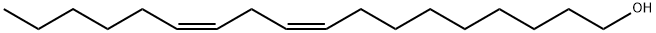
506-43-4
171 suppliers
$13.00/100mg
Yield:506-43-4 91%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuranReflux;
Steps:
1.1 (9z,12z)-Octadecadien-1-ol
Lithium aluminum hydride (2.73 g, 72 mmol) was suspended in tetrahydrofuran (THF, 190 mL) cooled at 4° C. Linoleic acid (10 g, 36 mmol) was added dropwise to the suspension, and the resulting mixture was stirred for 10 minutes. Then, the mixture was refluxed overnight with heating on an oil bath. After cooling the mixture, 1 mol/L aqueous sodium hydroxide (100 mL) was added to terminate the reaction. Then, the reaction mixture was diluted with ethyl acetate (100 mL), and filtered, and the filtrate was washed with saturated aqueous sodium hydrogencarbonate. Then, the organic layer was collected, and dried over anhydrous sodium sulfate. The organic layer was filtered, and the solvent was evaporated by using a rotating evaporator to obtain a crude product. The crude product was purified by silica gel chromatography (elution solvent, hexane:ethyl acetate, continuous gradient) to obtain 9z,12z-octadecadien-1-ol (8.68 g, 32.6 mmol) as colorless oil. Yield was 91%. Proton nuclear magnetic resonance (1H NMR, 500 MHz) data of 9z,12z-octadecadien-1-ol δ=0.88 (t, 3H), 1.25-1.36 (m, 16H), 1.53-1.58 (m, 2H), 2.02-2.06 (m, 4H), 2.76 (t, 2H), 3.62 (t, 2H), 5.29-5.40 (m, 4H)
References:
NATIONAL UNIVERSITY CORPORATION HOKKAIDO UNIVERSITY;Harashima, Hideyoshi;Sato, Yusuke;Warashina, Shota;Hatakeyama, Hiroto;Hyodo, Mamoru;Nakamura, Takashi US2017/273905, 2017, A1 Location in patent:Paragraph 0086-0087
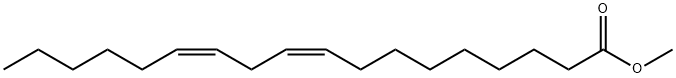
112-63-0
273 suppliers
$15.00/5g
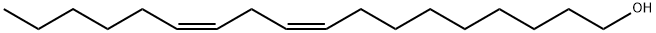
506-43-4
171 suppliers
$13.00/100mg

29204-30-6
9 suppliers
inquiry
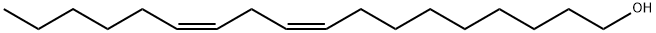
506-43-4
171 suppliers
$13.00/100mg

537-40-6
128 suppliers
$30.00/100mg
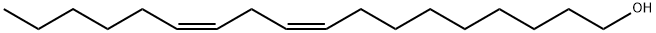
506-43-4
171 suppliers
$13.00/100mg
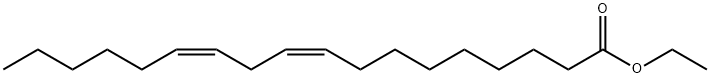
544-35-4
268 suppliers
$10.00/50mg
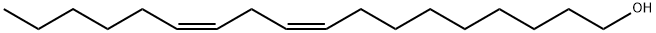
506-43-4
171 suppliers
$13.00/100mg