
TERT-BUTYL 4-HYDROXYBUTYRATE synthesis
- Product Name:TERT-BUTYL 4-HYDROXYBUTYRATE
- CAS Number:59854-12-5
- Molecular formula:C8H16O3
- Molecular Weight:160.21
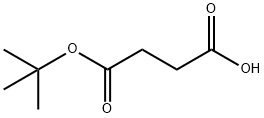
15026-17-2

59854-12-5
General procedure for the synthesis of tert-butyl 4-hydroxybutyrate from mono-tert-butyl succinate: BH3-Me2S (2.0 M solution in THF, 6.6 mL, 13.1 mmol) was slowly added dropwise to a solution of mono-tert-butyl succinate (2.12 g, 12.2 mmol) in anhydrous THF (20 mL) that was cooled to 0 °C. The reaction mixture was gradually warmed to room temperature and stirred continuously for 24 hours. Upon completion of the reaction, EtOAc (100 mL) was added for dilution and the organic layer was separated. The organic layer was washed sequentially with water (70 mL) and saturated saline (70 mL) and dried with anhydrous MgSO4. After filtration, the solvent was removed by concentration under reduced pressure to afford tert-butyl 4-hydroxybutyrate (1.92 g, 12.2 mmol, quantitative yield), the product was a light yellow oil, which could be used in the subsequent reaction without further purification.

15026-17-2
152 suppliers
$6.00/1g

59854-12-5
109 suppliers
$100.00/100 mg
Yield:59854-12-5 100%
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran at 0 - 20; for 24 h;Inert atmosphere;
Steps:
tert-Butyl 4-hydroxybutanoate, 9
BH3.Me2S (2.0 M in THF, 6.6 mL, 13.1 mmol) was added dropwise to a solution of carboxylic acid 8 (2.12 g, 12.2 mmol) in dry THF (20 mL) cooled to 0° C. The solution was allowed to warm to room temperature and stirred for 24 h. EtOAc (100 mL) was added and the organic layer separated and washed with water (70 mL) and brine (70 mL), dried over MgSO4, filtered and the solvent removed under reduced pressure to give tert-butyl 4-hydroxybutanoate 9 (1.92 g, 12.2 mmol, quant.), which was used without any further purification as a pale yellow oil
References:
Nortcliffe, Andrew;Fleming, Ian N.;Botting, Nigel P.;O'Hagan, David [Tetrahedron,2014,vol. 70,# 44,p. 8343 - 8347] Location in patent:supporting information
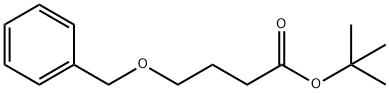
611212-49-8
1 suppliers
inquiry

59854-12-5
109 suppliers
$100.00/100 mg
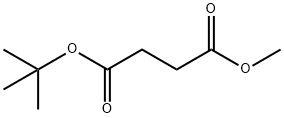
14734-25-9
25 suppliers
$96.00/5g

59854-12-5
109 suppliers
$100.00/100 mg
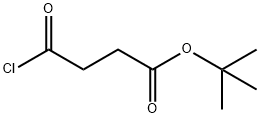
1025928-23-7
0 suppliers
inquiry

59854-12-5
109 suppliers
$100.00/100 mg
59854-06-7
0 suppliers
inquiry

59854-12-5
109 suppliers
$100.00/100 mg