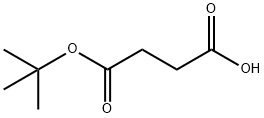
Mono-tert-butyl succinate synthesis
- Product Name:Mono-tert-butyl succinate
- CAS Number:15026-17-2
- Molecular formula:C8H14O4
- Molecular Weight:174.19

108-30-5
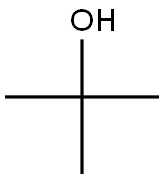
75-65-0

15026-17-2
Tert-butanol (10 mL) was added to a solution of toluene (100 mL) containing succinic anhydride (6.04 g, 60.40 mmol), N-hydroxysuccinimide (2.53 g, 22.01 mmol) and 4-dimethylaminopyridine (DMAP, 0.88 g, 7.23 mmol). The reaction mixture was heated under reflux conditions for 48 hours. Upon completion of the reaction, it was cooled to room temperature and two layers (brown oil and a clarified colorless solution) were observed to form in the reaction vessel. The crude product solution was diluted with ethyl acetate (EtOAc, 50 mL) and washed sequentially with 10% aqueous citric acid (2 x 50 mL) and saturated saline. The organic layer was dried over anhydrous sodium sulfate (Na2SO4) and the solvent was evaporated under reduced pressure. The crude product was recrystallized with a solvent mixture of ethyl ether/petroleum ether (1:3, 25 mL) to obtain mono-tert-butyl succinate in quantitative yield.

108-30-5
674 suppliers
$5.00/25g

75-65-0
784 suppliers
$10.00/10ml

15026-17-2
153 suppliers
$6.00/1g
Yield:15026-17-2 100%
Reaction Conditions:
with dmap;1-hydroxy-pyrrolidine-2,5-dione in toluene for 48 h;Reflux;
Steps:
4-(tert-Butoxy)-4-oxobutanoic Acid
The monoester of succinate was prepared as described by Srinivasan,Uttamchandani and Yao [42]: Tert-butanol (10 mL) was added to asolution of succinic anhydrate (6.04 g, 60.40 mmol), Nhydroxysuccinimid (2.53g, 22.01 mmol) and DMAP (0.88 g, 7.23 mmol) in toluene (100 mL)and the solution was heated for 48 h under reflux conditions. After cooling down to rt, twolayers formed in the reaction vessel (brown oil and clear, colourless solution). The crudesolution was diluted with EtOAc (50 mL) and washed with citric acid (10 %, 2 × 50 mL) andbrine. The organic layer was dried over Na2SO4, the solvent evaporated and the crude productwas recrystallized from Et2O/PE (1:3, 25 mL) to yield the title compound in quantitativeyield.
References:
Wünsch, Matthias;Schr?der, David;Fr?hr, Tanja;Teichmann, Lisa;Hedwig, Sebastian;Janson, Nils;Belu, Clara;Simon, Jasmin;Heidemeyer, Shari;Holtkamp, Philipp;Rudlof, Jens;Klemme, Lennard;Hinzmann, Alessa;Neumann, Beate;Stammler, Hans-Georg;Sewald, Norbert [Beilstein Journal of Organic Chemistry,2017,vol. 13,p. 2428 - 2441] Location in patent:supporting information
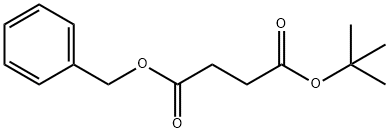
15026-16-1
1 suppliers
inquiry

15026-17-2
153 suppliers
$6.00/1g
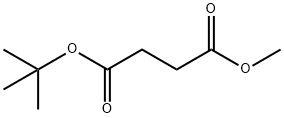
14734-25-9
25 suppliers
$96.00/5g

15026-17-2
153 suppliers
$6.00/1g
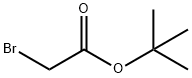
5292-43-3
453 suppliers
$10.00/5g

15026-17-2
153 suppliers
$6.00/1g
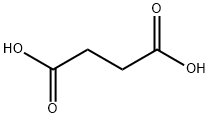
110-15-6
1007 suppliers
$5.00/10g

75-65-0
784 suppliers
$10.00/10ml

15026-17-2
153 suppliers
$6.00/1g