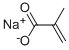
SODIUM METHACRYLATE synthesis
- Product Name:SODIUM METHACRYLATE
- CAS Number:5536-61-8
- Molecular formula:C4H5NaO2
- Molecular Weight:108.07
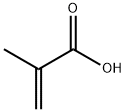
79-41-4
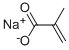
5536-61-8
Example 2 Preparation of sodium methacrylate: 12.23 g (0.3058 mol) of sodium hydroxide sample was placed in a 50 ml conical flask containing 10 ml of deionized water and a magnetic stirrer and stirred until completely dissolved. Subsequently, the sodium hydroxide solution was slowly added to a 250 ml beaker containing 30.70 g (0.3570 mol) of methacrylic acid under continuous stirring conditions (note: this process is exothermic). After the reaction mixture was cooled to room temperature, 50 ml of acetone was added to promote precipitation of the product. The precipitate was collected by vacuum filtration. The resulting carboxylate was initially air-dried in a fume hood and subsequently transferred to an oven and dried at 55°C to 60°C for 12 to 15 hours to yield 30.43 g of sodium methacrylate in 92.2% yield.

79-41-4
533 suppliers
$14.00/5g
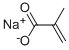
5536-61-8
300 suppliers
$6.00/10g
Yield:5536-61-8 92.2%
Reaction Conditions:
with sodium hydroxide in water;acetone
Steps:
2 Example 2
Example 2 Preparation of Sodium Methacrylate A 12.23 g (0.3058 mol) sample of sodium hydroxide was placed in a 50-ml Erlenmayer flask equipped with 10 ml water and a magnetic stirring bar, and the mixture was stirred to dissolution. The sodium hydroxide solution was then poured into a 250-ml beaker containing 30.70 g (0.3570 mol) methacrylic acid (exothermic reaction) with constant stirring. The mixture was allowed to cool, 50 ml of acetone were added, and the precipitate vacuum filtered. The recovered carboxylate salt was first allowed to air dry under a hood, and then dried in an oven at 55°-60° C. for 12-15 hours to afford 30.43 g (92.2% yield) of sodium methacrylate.
References:
Howard University US5491244, 1996, A
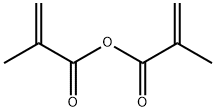
760-93-0
331 suppliers
$27.60/50g
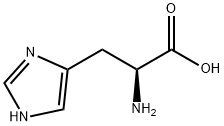
71-00-1
802 suppliers
$7.00/5g
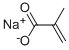
5536-61-8
300 suppliers
$6.00/10g
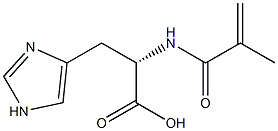
13282-13-8
1 suppliers
inquiry