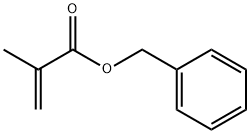
Benzyl methacrylate synthesis
- Product Name:Benzyl methacrylate
- CAS Number:2495-37-6
- Molecular formula:C11H12O2
- Molecular Weight:176.21
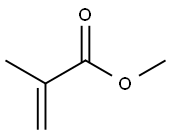
80-62-6
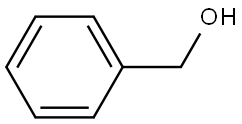
100-51-6

2495-37-6
GENERAL METHODS: In Examples 62-64, methyl methacrylate was converted to benzyl methacrylate with benzyl alcohol by an ester exchange reaction as shown in Table 13. In Example 62, lanthanum nitrate and tri-n-octylphosphine were first azeotropically refluxed with dimethyl carbonate for 1 hour according to the method of Example 24, followed by evaporation of the dimethyl carbonate at room temperature, and the resulting catalyst was used in the reaction. In Examples 63 and 64, methyltridecylammonium methyl carbonate was used directly as the catalyst. Under these conditions, benzyl methacrylate was obtained in high yields. It is to be noted that in Example 62, higher yields than Example 62 are expected to be obtained if isolated phosphonium salts are used rather than in situ prepared catalysts. Although not listed in Table 13, a variety of by-products are generated when potassium tert-butoxide is used as a catalyst.

80-62-6
626 suppliers
$18.00/25mL

100-51-6
1446 suppliers
$5.00/100g

2495-37-6
307 suppliers
$6.00/25g
Yield:2495-37-6 97%
Reaction Conditions:
with C2H3O3(1-)*C37H78N(1+) in hexane at 90; for 2 h;Molecular sieve;Reagent/catalyst;
Steps:
63
General procedure: In Examples 62 to 64, as shown in Table 13, a transesterification reaction was carried out between methyl methacrylate which is an easily polymerizable ester compound and an alcohol compound. In Example 62, as in Example 24, lanthanum nitrate and tri n-octylphosphine were previously azeotropically refluxed with dimethyl carbonate for 1 hour, then dimethyl carbonate was evaporated at room temperature, The obtained catalyst was used. In Examples 63 and 64, methyltridodecylammonium methyl carbonate was used singly. As a result, ester products were obtained in high yield. It is to be noted that in Example 62, when using the isolated phosphonium salt as a catalyst instead of using the prepared catalyst as it is, it is predicted that the ester product is obtained with a higher yield than in Example 62 . Although not shown in Table 13, potassium tert-butoxide was used as a catalyst, and various by-products were produced.
References:
NAGOYA UNIVERSITY;MITSUBISHI RAYON COMPANY LIMITED;SHIHARA, KAZUAKI;HATANO, MANABU JP5804472, 2015, B2 Location in patent:Paragraph 0087; 0088
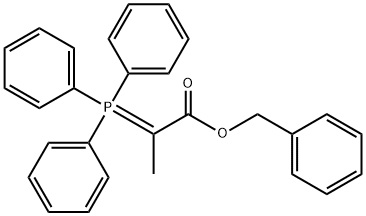
63613-50-3
0 suppliers
inquiry

2495-37-6
307 suppliers
$6.00/25g
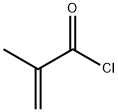
920-46-7
341 suppliers
$20.00/1g

100-51-6
1446 suppliers
$5.00/100g

2495-37-6
307 suppliers
$6.00/25g
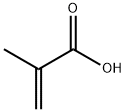
79-41-4
533 suppliers
$14.00/5g

100-51-6
1446 suppliers
$5.00/100g

2495-37-6
307 suppliers
$6.00/25g
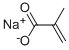
5536-61-8
301 suppliers
$6.00/10g

100-39-0
438 suppliers
$10.00/10 g

2495-37-6
307 suppliers
$6.00/25g