
Pepstatin synthesis
- Product Name:Pepstatin
- CAS Number:26305-03-3
- Molecular formula:C34H63N5O9
- Molecular Weight:685.89
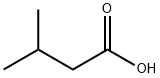
503-74-2
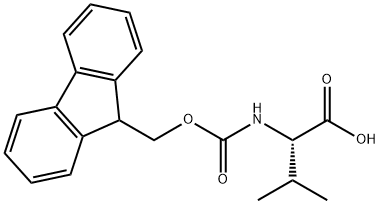
68858-20-8
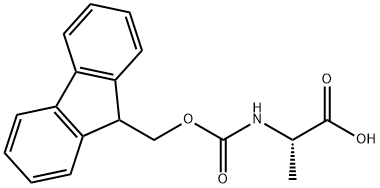
35661-39-3
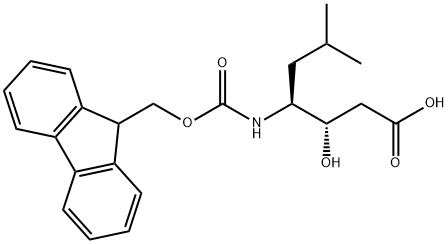
158257-40-0

26305-03-3
General steps: 4.2 General Procedure A: Resin Loading Solid phase peptide synthesis was performed manually in a sintered polypropylene syringe. The 2-chlorotrityl chloride (CTC) resin was pre-immersed in dichloromethane (DCM) for 15 minutes and drained. The first amino acid in a DCM solution of 0.4 M N,N-diisopropylethylamine (DIPEA) was added and the mixture was stirred for 3 hours. After draining the solvent, the resin was treated with a 17:2:1 DCM/methanol (MeOH)/DIPEA (3 × 3 mL × 5 min) solution, and then any free 2-CTC resin joints were blocked with an 8:1:1 N,N-dimethylformamide (DMF)/DIPEA/acetic anhydride (2 × 3 mL × 10 min) solution. The resin was finally washed with DCM (2 × 3 mL × 1 min), DMF (2 × 3 mL × 1 min), DCM (2 × 3 mL × 1 min), and DMF (2 × 3 mL × 1 min). 4.3 General method B: Fmoc deprotection The resin was stirred with a 10% piperidine solution of DMF (2 x 3mL x 3 min) and subsequently washed with DMF (3 x 3mL x 1 min), DCM (3 x 3mL x 1 min), and DMF (5 x 3mL x 1 min). The deprotected solutions were combined and diluted appropriately (100-fold, 0.05 mmol resin loading). Resin loading was estimated by measuring the absorbance of piperidine-fulvene adduct with 10% piperidine in DMF as reference (λ = 301 nm; ε = 7800 M-1cm-1). 4.4 Generalized method C: peptide coupling with HBTUs Solutions of amino acids protected by appropriate Fmoc (3 eq. relative to resin loading) and 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) (2.9 eq. relative to resin loading) were prepared in minimal amounts of DMF. DIPEA (6 equivalents relative to resin loading) was added and the resin was stirred for 1.5 hours. The resin was then drained and washed with DMF (3 x 3 mL x 1 min), DCM (3 x 3 mL x 1 min) and DMF (5 x 3 mL x 1 min). 4.5 Generalized Method D: Peptide Coupling with HATUs Solutions of amino acids protected by appropriate Fmoc (3 equivalents relative to resin loading) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate (HATU) (2.9 equivalents relative to resin loading) were prepared in minimal DMF. DIPEA (6 equiv. vs. resin loading) was added to the solution and the mixture was immediately added to the resin and stirred. The reaction time was varied based on the coupled residues: Phe (NMe) and Ala (2 × 2 h); Thr and Sta (1 × 2 h); Asn, Leu, D-Val, and L-Val (2 × 2 h); and DMVal (3 × 3 h). Once the reaction was complete, the resin was drained and washed with DMF (3 × 3 mL × 1 min), DCM (3 × 3 mL × 1 min), and DMF (3 × 3 mL × 1 min). 4.6 Generalized Method E: Peptide Coupling to DICs A solution of amino acids protected by appropriate Fmoc (1.5 equivalents relative to resin loading), 1-hydroxybenzotriazole (HOBt) (1.5 equivalents relative to resin loading), and N,N'-diisopropylcarbodiimide (DIC) (1.5 equivalents relative to resin loading) in minimal DMF was prepared. The solution was stirred for 20 minutes, then added to the resin and stirred overnight. The resin was drained and washed with DMF (3 × 3 mL × 1 min), DCM (3 × 3 mL × 1 min), and DMF (5 × 3 mL × 1 min). Application of coupled fluorinated amino acids followed by double coupling of the next amino acid. 4.7 Generalized Procedure F: Resin Cleavage After final Fmoc deprotection, the resin was washed with DMF (3 x 3mL x 1 min) and DCM (3 x 3mL x 1 min) and then vacuum dried. The resin was stirred with 95:2.5:2.5 trifluoroacetic acid (TFA)/triisopropylsilane (TIS)/H2O (3 mL) solution for 2 hours. The resin was drained and washed with the same TFA mixture described above (2 × 3 mL × 1 min). The combined lysis solutions were concentrated under a stream of nitrogen. Ether was added and the supernatant was decanted off (3×). The residue was then dried under vacuum to give a crude linear peptide.

503-74-2
439 suppliers
$5.00/10g

68858-20-8
544 suppliers
$8.00/1g

35661-39-3
539 suppliers
$5.00/1g

158257-40-0
171 suppliers
$11.00/100mg

26305-03-3
313 suppliers
$24.00/1mg
Yield:26305-03-3 46%
Reaction Conditions:
Stage #1:(3S,4S)-(9-fluorenylmethoxycarbonyl)-4-amino-3-hydroxy-6-methylheptanoic acid with N-ethyl-N,N-diisopropylamine in dichloromethane for 3 h;
Stage #2: with piperidine in N,N-dimethyl-formamide for 0.1 h;
Stage #3:3-methylbutyric acid;Fmoc-Val-OH;N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-alanine;(3S,4S)-(9-fluorenylmethoxycarbonyl)-4-amino-3-hydroxy-6-methylheptanoic acidFurther stages;
Steps:
General procedure: 4.2 General procedure A: resin loading (0026) Solid phase peptide synthesis was conducted manually in a sinter-fitted polypropylene syringe. 2-Chlorotritylchloride (CTC) resin was preswelled in DCM (mL) for 15min and drained. The first amino acid in 0.4M DIPEA/DCM was added and the mixture was agitated for 3h. After draining the solvent, any free 2-CTC resin linkers were capped by treatment of the resin with a solution of 17:2:1 DCM/MeOH/DIPEA (3×3mL×5min), and subsequently with a solution of 8:1:1 DMF/DIPEA/acetic anhydride (2×3mL×10min). The resin was finally washed with DCM (2×3mL×1min), DMF (2×3mL×1min), DCM (2×3mL×1min) and DMF (2×3mL×1min). 4.3 General procedure B: Fmoc deprotection (0027) The resin was agitated with a solution of 10% piperidine inDMF (2×3mL×3min) and subsequently washed with DMF(3×3mL×1min), DCM (3×3mL×1min), DMF (5×3mL×1min). The deprotected solutions were combined and diluted appropriately (100-fold for 0.05mmol resin loading). The resin loading was estimated by measuring the absorbance of the piperidine-fulvene adduct with 10% piperidine in DMF as a reference (λ=301nm; ε=7800M-1cm-1). 4.4 General procedure C: peptide coupling with HBTU (0028) A solution was prepared of the appropriate Fmoc-protected amino acid (3 equiv. relative to resin loading) and HBTU (2.9 equiv. relative to resin loading) in minimum amount of DMF. DIPEA (6 equiv. relative to resin loading) was added and the resin was agitated for 1.5h. The resin was then drained and washed with DMF (3×3mL×1min), DCM (3×3mL×1min) and DMF (5×3mL×1min). 4.5 General procedure D: peptide coupling with HATU (0029) A solution was prepared of the appropriate Fmoc-protected amino acid (3 equiv. relative to resin loading) and HATU (2.9 equiv. relation to resin loading) in minimal DMF. DIPEA (6 equiv. relation to resin loading) was added to the solution and the mixture was immediately added to the resin and agitated. Reaction times were altered based on the residue being coupled: Phe(NMe) and Ala (2×2h); Thr and Sta (1×2h); Asn, Leu, D-Val and L-Val (2×2h); DMVal (3×3h). Once the reaction was complete, the resin was drained and washed with DMF (3×3mL×1min), DCM (3×3mL×1min) and DMF (3×3mL×1min). 4.6 General procedure E: peptide coupling with DIC (0030) A solution was prepared of the appropriate Fmoc-protected amino acid (1.5 equiv. relative to resin loading), HOBt (1.5 equiv. relative to resin loading) and DIC (1.5 equiv. relative to resin loading) in minimal DMF. This solution was stirred for 20min, then added to the resin and agitated overnight. The resin was drained and washed with DMF (3×3mL×1min), DCM (3×3mL×1min) and DMF (5×3mL×1min). Double coupling of the next amino acid after the coupling of the fluorinated amino acid was applied. 4.7 General procedure F: resin cleavage (0031) After the last Fmoc deprotection, the resin was washed with DMF (3×3mL×1min) and DCM (3×3mL×1min) then dried in vacuo. The resin was agitated with a solution of 95:2.5:2.5 TFA/TIS/H2O (3mL) for 2h. The resin was drained and washed with the same TFA mixture above (2×3mL×1min). The combined cleavage solutions were concentrated under a stream of nitrogen. Diethyl ether was added and the supernatant was decanted (3×). The residue was then dried in vacuo to provide the crude linear peptide
References:
Lawer, Aggie;Nesvaderani, John;Marcolin, Gabriella M.;Hunter, Luke [Tetrahedron,2018,vol. 74,# 12,p. 1278 - 1287]