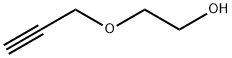
Propynol ethoxylate synthesis
- Product Name:Propynol ethoxylate
- CAS Number:3973-18-0
- Molecular formula:C5H8O2
- Molecular Weight:100.12
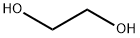
107-21-1
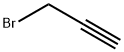
106-96-7

3973-18-0
4.2.2. Synthesis of 2-(prop-2-ynyloxy)ethanol (2). Under argon protection, propargyl bromide (2.5 mL, 80% toluene solution, 22.5 mmol) was mixed with ethylene glycol (2.5 mL) and cooled to 0 °C in an ice bath. Powdered NaOH (1.08 g, 45.0 mmol) was slowly added to the reaction system, followed by warming the reaction mixture to 45 °C and stirring for 3 hours. After completion of the reaction, the precipitate was removed by filtration and washed with dichloromethane (23 mL). The filtrates were combined and extracted with dichloromethane (310 mL). The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=2:1) and after evaporation of the solvent under reduced pressure, 1.34 g (60% yield) of target compound 2 was obtained as a light yellow oil. Thin layer chromatography Rf value 0.25 (Expanding agent: hexane/ethyl acetate=2:1). IR spectrum (neat, cm?1): 1106, 1246, 1729, 2115, 2868, 2935, 3287, 3400. 1H NMR (CDCl?, 250 MHz): δ 2.42 (1H, t, J=2.2 Hz), 2.99 (1H, s), 3.55 (2H, t, J=4.4 Hz), 3.66 (2H, t, J=4.4 Hz), 4.12 (2H, d, J=2.2 Hz).13C NMR (CDCl?, 62.5 MHz): δ 58.1, 61.2, 71.0, 74.6, 79.3.High-resolution mass spectra (ESI): [M+H]? Calculated C?H?O?: 101.0603, measured: 101.0602.
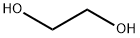
107-21-1
1374 suppliers
$10.00/25g

106-96-7
343 suppliers
$31.00/25g

3973-18-0
231 suppliers
$31.80/1gm:
Yield:3973-18-0 80%
Reaction Conditions:
Stage #1: ethylene glycolwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.5 h;
Stage #2: propargyl bromide at 0 - 50;
Steps:
1 Step 1:
2-(prop-2-yn-1-yloxy)ethan-1-ol
To a stirred mixture of sodium hydride (60% in mineral oil, 115 mg, 2.8 mmol) in anhydrous N,N-dimethylformamide (20 ml) at 0° C. was added ethane-1,2-diol (3.9 g, 63 mmol) and stirred at 0° C. for 0.5 hour.
To the resulting mixture was added 3-bromoprop-1-yne (5.0 g, 42 mmol) at 0° C. and stirred at 50° C. overnight. TLC showed the reaction was complete.
The reaction mixture was quenched with ice water (20 ml) and partitioned between ethyl acetate (80 ml) and water (100 ml).
The organic layer was collected, and the aqueous layer was extracted with ethyl acetate (50 ml*2).
The combined organic layers were washed with brine (80 ml), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give a crude residue which was purified by silica gel flash chromatography (eluted with 0-20% ethyl acetate in hexane) to afford 2-(prop-2-yn-1-yloxy)ethanol (3.4 g, yield 80%) as colorless oil.
References:
US2018/125821,2018,A1 Location in patent:Paragraph 0858; 0859
![Silane, (1,1-dimethylethyl)dimethyl[2-(2-propyn-1-yloxy)ethoxy]-](/CAS/20210305/GIF/339531-56-5.gif)
339531-56-5
1 suppliers
inquiry

3973-18-0
231 suppliers
$31.80/1gm:
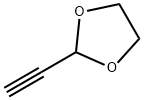
18938-38-0
0 suppliers
inquiry

3973-18-0
231 suppliers
$31.80/1gm:

106-96-7
343 suppliers
$31.00/25g

3973-18-0
231 suppliers
$31.80/1gm:
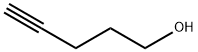
5390-04-5
287 suppliers
$13.00/1g

540-51-2
258 suppliers
$17.00/5g

3973-18-0
231 suppliers
$31.80/1gm: