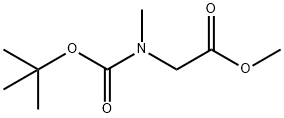
N-Boc-N-methyl glycine methyl ester synthesis
- Product Name:N-Boc-N-methyl glycine methyl ester
- CAS Number:42492-57-9
- Molecular formula:C9H17NO4
- Molecular Weight:203.24

24424-99-5

13515-93-0

42492-57-9
Thionyl chloride (29.02 mL, 0.40 mol) was slowly added dropwise to a suspension of sarcosine methyl ester hydrochloride (35.64 g, 0.40 mol) in 350 mL of methanol at 0 °C. After completion of the dropwise addition, the ice bath was removed and the reaction mixture was stirred at room temperature for 30 minutes followed by reflux for 6 hours. Upon completion of the reaction, the solution was concentrated under reduced pressure and the residue was dried overnight under high vacuum to afford sarcosine methyl ester hydrochloride in the form of a white powder, which could be used in the next step of the reaction without further purification. Yield: 56.14 g (quantitative); Melting point: 102°C. NMR hydrogen spectrum (300 MHz, CDCl3): δ=9.76 (s, 2H, NH2), 3.88 (t, J=5.6 Hz, 2H, CH2), 3.82 (s, 3H, OCH3), 2.83 (t, J=5.2 Hz, 3H, NCH3). NMR carbon spectrum (75MHz, CDCl3): δ=166.7 (C=O), 53.3 (CH2), 48.9 (NCH3), 33.4 (OCH3). Sarcosine methyl ester hydrochloride (59.80 g, 0.43 mol) was suspended in 1.0 L of dichloromethane, and a mixture of triethylamine (119.09 mL, 0.86 mol) and di-tert-butyl dicarbonate (138.01 mL, 0.65 mol) was added slowly and dropwise at 0 °C. After dropwise addition, the ice bath was removed and the reaction mixture was stirred at room temperature for 2 days. After completion of the reaction, the reaction was adjusted to pH=6 by adding 1 M aqueous hydrochloric acid, the organic layer was separated, washed with distilled water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and volatile impurities were removed overnight under high vacuum to give the clarified oily product methyl 2-((tert-butoxycarbonyl)(methyl)amino)acetate. Yield: 78.65 g (90%).

24424-99-5
867 suppliers
$13.50/25G

13515-93-0
291 suppliers
$10.00/5G

42492-57-9
86 suppliers
$15.00/1g
Yield: 90%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 48 h;Inert atmosphere;
Steps:
N-tert-Butoxycarbonyl-sarcosine methyl ester 2
To a suspension of sarcosine (35.64 g, 0.40 mol) in 350 mL MeOH was added thionyl chloride (29.02 mL, 0.40 mol) dropwise at 0 °C. After complete addition the cooling bath was removed and the reaction mixture was stirred for 30 min at r.t. and refluxed for further 6 h. The resulting solution was concentrated in vacuum and the residue was dried in high vacuum over night at r.t. to afford sarcosine methyl ester.HCl as a white powder which was used in the next step without further purification; yield: 56.14 g (quant.); mp. 102 °C. 1H-NMR (300 MHz, CDCl3): δ = 9.76 (s, 2 H, NH2), 3.88 (t, J = 5.6 Hz, 2 H, H-2), 3.82 (s, 3 H, H-4)), 2.83 (t, J = 5.2 Hz, 3 H, H-3). 13C-NMR(75 MHz, CDCl3): δ = 166.7 (C-1), 53.3 (C-2), 48.9 (C-3), 33.4 (C-4).1 To a suspension of sarcosine methylester.HCl (59.80 g, 0.43 mol) and (Boc)2O (138.01 mL, 0.65 mol) in 1.0 L CH2Cl2 TEA (119.09 mL, 0.86 mol) was added dropwise at 0 °C. After complete addition the cooling bath was removed and the reaction mixture was stirred for two days at r.t. Aq. HCl (1 M) was added until the aqueous layer showed pH = 6. The resulting two layers were separated and the organic layer was washed with dist. H2O, dried (Na2SO4), concentrated in vacuum and volatile impurities were removed in high vacuum over night at r.t. to afford a clear oil; yield: 78.65 g (90 %)
References:
Przybyla, Daniel;Nubbemeyer, Udo [Synthesis,2017,vol. 49,# 4,art. no. SS-2016-Z0507-OP,p. 770 - 774] Location in patent:supporting information
13734-36-6
308 suppliers
$11.19/5G

74-88-4
356 suppliers
$15.00/10g

42492-57-9
86 suppliers
$15.00/1g
31954-27-5
239 suppliers
$10.00/1G

74-88-4
356 suppliers
$15.00/10g

42492-57-9
86 suppliers
$15.00/1g

13734-36-6
308 suppliers
$11.19/5G
6674-22-2
641 suppliers
$5.00/10g

74-88-4
356 suppliers
$15.00/10g

42492-57-9
86 suppliers
$15.00/1g

13734-36-6
308 suppliers
$11.19/5G
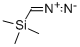
18107-18-1
210 suppliers
$33.00/5g

42492-57-9
86 suppliers
$15.00/1g