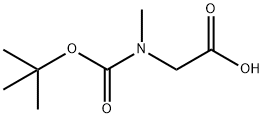
BOC-SAR-OH synthesis
- Product Name:BOC-SAR-OH
- CAS Number:13734-36-6
- Molecular formula:C8H15NO4
- Molecular Weight:189.21

107-97-1

24424-99-5

13734-36-6
Triethylamine (23.5 mL, 168.3 mmol) and di-tert-butyl dicarbonate (12.9 mL, 56.1 mmol) were sequentially added to an aqueous (375 mL) solution of sarcosine (5.0 g, 56.1 mmol), and the reaction mixture was stirred for 6 hours at room temperature. After completion of the reaction, hydrochloric acid solution (320 mL, 1 M aqueous solution) and ethyl acetate were added for acidification and extraction. After separation of the aqueous layer, the aqueous layer was further extracted with ethyl acetate. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give a colorless oil. The oily substance was crystallized at -15 °C to afford the target product 2-((tert-butoxycarbonyl)(methyl)amino)acetic acid (10.6 g, quantitative yield) as a white solid and a mixture of rotary isomers was observed.
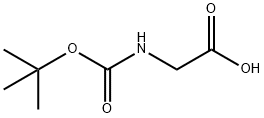
4530-20-5
571 suppliers
$5.00/5 g

74-88-4
358 suppliers
$15.00/10g

13734-36-6
309 suppliers
$11.19/5G
Yield:13734-36-6 94%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 0 - 20; for 26 h;Inert atmosphere;
Steps:
Experimental procedure for the synthesisof intermediates (6a-6i)
General procedure: (S)-2-(Tert-butoxycarbonyl(methyl)amino)-3-hydroxypropanoic acid(6a) Gummy substance (This compound was prepared byadding neat sodium hydride (10 equiv.) in portion wiseover a period of 2.0 h to a cooled (0 °C) solution of (S)-2-(tert-butoxycarbonylamino)-3-hydroxypropanoic acid (1equiv.) and iodomethane (10 equiv.) in dry THF under astream of nitrogen. The reaction mixture was stirred at room temperature for 24 h under nitrogen atmosphere andthen diluted with ether (20 mL) and quenched with water(30 mL). The layers were separated and the aqueous layerwas extracted with ether (2 x9 15 mL), acidified to pH 3with a 20 % aqueous solution of citric acid and extractedwith EtOAc (3 x 20 mL). The combined organic phasewas dried over Na2SO4 and evaporated to afford thecorresponding N-methylated product in 90 % yield asGummy substance.)
References:
Srinivasarao, Kondaparla;Agarwal, Pooja;Srivastava, Kumkum;Haq;Puri, Sunil K.;Katti [Medicinal Chemistry Research,2016,vol. 25,# 6,p. 1148 - 1162]

107-97-1
602 suppliers
$5.00/100mg

24424-99-5
866 suppliers
$13.50/25G

13734-36-6
309 suppliers
$11.19/5G
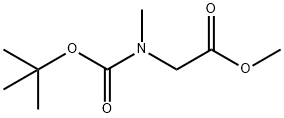
42492-57-9
86 suppliers
$15.00/1g

13734-36-6
309 suppliers
$11.19/5G

107-97-1
602 suppliers
$5.00/100mg
58632-95-4
278 suppliers
$7.00/5g

13734-36-6
309 suppliers
$11.19/5G

24424-99-5
866 suppliers
$13.50/25G

13734-36-6
309 suppliers
$11.19/5G