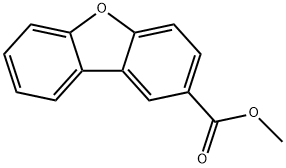
METHYL 2-DIBENZOFURANCARBOXYLATE synthesis
- Product Name:METHYL 2-DIBENZOFURANCARBOXYLATE
- CAS Number:58841-72-8
- Molecular formula:C14H10O3
- Molecular Weight:226.23

67-56-1
785 suppliers
$9.00/25ml
22439-48-1
35 suppliers
$57.50/250mg

58841-72-8
9 suppliers
$106.00/50mg
Yield:58841-72-8 650 mg
Reaction Conditions:
with thionyl chloride at 80; for 4 h;
Steps:
24.24b Step 24b: Preparation of diphenyl[b,d]furan-2-carboxylate (compound 0603-96):
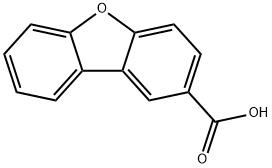
The compound diphenyl[b,d]furan-2-carboxylic acid (0602-96)(1.2g, 5.66 mmol, 1.0 eq)Dissolved in 50 ml of methanol,Cool in an ice bath and slowly add thionyl chloride (0.82 mL, 11.3 mmol, 2.0 equiv).Heat to 80°C and react for 4 hours.After the reaction was completed, it was concentrated under reduced pressure.Dissolve the residue in twoIn methyl chloride, saturated sodium bicarbonate solution was added and the solution was made alkaline to separate the solution. The organic phase was dried over anhydrous sodium sulfate, and spin-dry. The residue was purified by silica gel column chromatography (eluent: ethyl acetate/petroleum ether=1/5) to give the product dibenzo[b,d]furan-2-one carboxylic acidMethyl ester (650 mg, yield: 55%).
References:
CN107383024,2017,A Location in patent:Paragraph 0279
15126-06-4
140 suppliers
$6.00/250mg
88284-48-4
169 suppliers
$9.00/250mg

58841-72-8
9 suppliers
$106.00/50mg
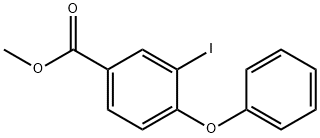
100725-29-9
17 suppliers
$12.00/1mg

58841-72-8
9 suppliers
$106.00/50mg
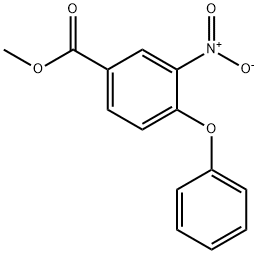
73938-97-3
0 suppliers
inquiry

58841-72-8
9 suppliers
$106.00/50mg

15126-06-4
140 suppliers
$6.00/250mg

58841-72-8
9 suppliers
$106.00/50mg