
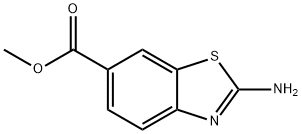
6-Benzothiazolecarboxylicacid,methylester(9CI) synthesis
- Product Name:6-Benzothiazolecarboxylicacid,methylester(9CI)
- CAS Number:73931-63-2
- Molecular formula:C9H7NO2S
- Molecular Weight:193.22
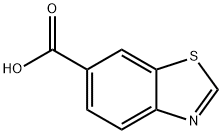
3622-35-3
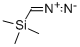
18107-18-1

73931-63-2
1. 1,3-Benzothiazole-6-carboxylic acid (5.0 g, 27.93 mmol) was dissolved in a solvent mixture of dichloromethane (96 mL) and methanol (32 mL) and the solution was cooled to 0°C. 2. A hexane solution of trimethylsilylated diazomethane (28 mL, 2.0 M) was slowly added dropwise with stirring. 3. After the dropwise addition is complete, the reaction mixture is gradually warmed to room temperature and stirring is continued overnight. 4. Upon completion of the reaction, acetic acid (2 mL) was carefully added to quench the reaction and stirring was continued for 30 minutes. 5. The reaction solution was concentrated, diluted with ethyl acetate and washed with saturated sodium bicarbonate solution. 6. Separate the organic layer, dry with anhydrous sodium sulfate, filter and concentrate under reduced pressure. 7. The crude product was purified by silica gel column chromatography using 15% ethyl acetate/hexane as eluent to afford methyl 6-benzothiazolecarboxylate (4.44 g, 82% yield) as a white solid. 8. The structure of the product was determined by 1H2O2 chromatography. 8. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 9.15 (s, 1H), 8.68 (m, 1H), 8.16 (m, 2H), 3.97 (s, 3H).

3622-35-3
149 suppliers
$13.00/250mg
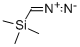
18107-18-1
210 suppliers
$33.00/5g

73931-63-2
55 suppliers
inquiry
Yield: 82%
Reaction Conditions:
Stage #1:benzothiazole-6-carboxylic acid;diazomethyl-trimethyl-silane in methanol;dichloromethane at 0 - 20;
Stage #2: with acetic acid in methanol;dichloromethane for 0.5 h;
Steps:
23.1
Benzothiazole-6-carboxylic acid (71a, 5.0 g, 27.93 mmol) was dissolved in DCM (96 mL) and MeOH (32 mL) and cooled to 0° C. A solution of trimethylsilyl-diazomethane (28 mL, 2.0M in hexane) was added dropwise and the resulting solution was gradually warmed to RT and stirred overnight. The reaction was quenched slowly by careful addition of HOAc (2 mL) and stirred for 30 min. The solution was concentrated, diluted with EtOAc and washed with saturated NaHCO3 solution. The organic extracts were dried (Na2SO4), filtered and concentrated in vacuo. The crude residue was purified by SiO2 chromatographed eluting with 15% EtOAc/hexane to afford 4.44 g (82%) of 71b as a white solid: 1H NMR (300 MHz, CDCl3): 9.15 (s, 1H), 8.68 (m, 1H), 8.16 (m, 2H), 3.97 (s, 3H).
References:
Roche Palo Alto LLC US2006/40927, 2006, A1 Location in patent:Page/Page column 38
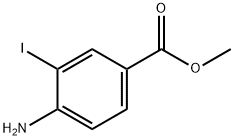
19718-49-1
123 suppliers
$8.00/1g
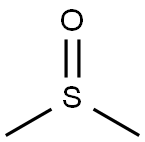
67-68-5
1310 suppliers
$15.00/100g

73931-63-2
55 suppliers
inquiry

67-56-1
785 suppliers
$7.29/5ml-f

3622-35-3
149 suppliers
$13.00/250mg

73931-63-2
55 suppliers
inquiry
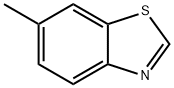
66947-92-0
119 suppliers
$6.00/100mg

73931-63-2
55 suppliers
inquiry
2942-15-6
45 suppliers
$45.00/5mg

73931-63-2
55 suppliers
inquiry