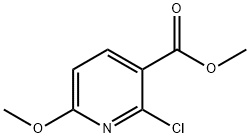
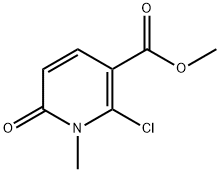
METHYL 2-CHLORO-6-METHOXYNICOTINATE synthesis
- Product Name:METHYL 2-CHLORO-6-METHOXYNICOTINATE
- CAS Number:95652-77-0
- Molecular formula:C8H8ClNO3
- Molecular Weight:201.61
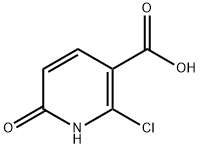
38025-90-0

74-88-4

95652-77-0
General Steps: 1. 2-Chloro-6-hydroxynicotinic acid (3.00 g, 17.3 mmol) and chloroform (30 mL) were added to a 50 mL round-bottomed flask and stirred until completely dissolved. 2. Silver carbonate (11.0 g, 39.8 mmol) and methyl iodide (3.77 mL, 60.5 mmol) were added sequentially to the above solution. 3. The reaction mixture was stirred at 50 °C for 3 hours and the reaction was monitored by TLC. 4. 4. After completion of the reaction, the mixture was vacuum filtered and the filter cake was washed with chloroform. 5. The filtrate and washings were combined and concentrated under reduced pressure in a rotary evaporator to give the crude product. 6. The crude product was purified by fast column chromatography (eluent: 0-100% ethyl acetate/heptane gradient elution) and the target fraction was collected. 7. The target fraction was concentrated under pressure to afford methyl 2-chloro-6-methoxypyridine-3-carboxylate (2.42 g, 69% yield) as a white powder. 8. The product was purified by LCMS (0-100% ethyl acetate/heptane gradient elution). 8. The product was characterized by LCMS (Method D): retention time 1.13 min, m/z = 202 (M + 1).

38025-90-0
125 suppliers
$10.00/100mg

74-88-4
356 suppliers
$15.00/10g

95652-77-0
100 suppliers
$82.00/100mg
Yield: 69%
Reaction Conditions:
with silver carbonate in chloroform at 50; for 3 h;
Steps:
1 Methyl 2-chloro-6-methoxypyridine-3-carboxylate (EV-AY8827-002)- Step 1
Methyl 2-chloro-6-methoxypyridine-3-carboxylate (EV-AY8827-002)- Step 1 Ag2CO3 (11.0 g, 39.8 mmol) and methyl iodide (3.77 ml, 60.5 mmol) were added to a solution of 2-chloro-6-oxo-1,6-dihydropyridine-3-carboxylic acid (CAS 1805670-73-8, 3.00 g, 17.3 mmol) in chloroform (30 ml) and the resulting mixture was stirred at 50°C for 3h. The reaction mixture was filtered under vacuum (washing with chloroform) and the filtrate was concentrated in vacuo. The residue was purified by flash column chromatography (0-100% ethyl acetate/heptane) to afford 2.42 g (69%) of methyl 2-chloro-6-methoxypyridine-3-carboxylate (EV-AY8827-002) as a white powder. LCMS (method D): retention time 1.13min, M/z = 202 (M + 1).
References:
PADLOCK THERAPEUTICS, INC.;DEVRAJ, Rajesh;KUMARAVEL, Gnanasambandam;ATTON, Holly;BEAUMONT, Edward;GADOULEAU, Elise;GLEAVE, Laura;KERRY, Philip Stephen;LECCI, Cristina;MENICONI, Mirco;MONCK, Nat;PALFREY, Jordan;PAPADOPOULOS, Kostas;TYE, Heather;WOODS, Philip A. WO2017/147102, 2017, A1 Location in patent:Paragraph 00376-00377
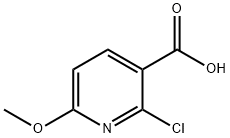
503000-87-1
91 suppliers
$37.00/250mg

74-88-4
356 suppliers
$15.00/10g

95652-77-0
100 suppliers
$82.00/100mg

67-56-1
782 suppliers
$7.29/5ml-f

503000-87-1
91 suppliers
$37.00/250mg

95652-77-0
100 suppliers
$82.00/100mg

503000-87-1
91 suppliers
$37.00/250mg
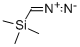
18107-18-1
210 suppliers
$33.00/5g

95652-77-0
100 suppliers
$82.00/100mg

74-88-4
356 suppliers
$15.00/10g
853109-24-7
15 suppliers
inquiry

95652-77-0
100 suppliers
$82.00/100mg