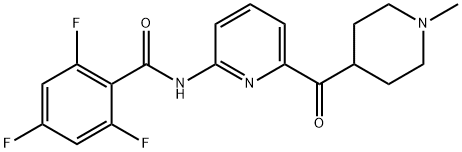
LasMiditan synthesis
- Product Name:LasMiditan
- CAS Number:439239-90-4
- Molecular formula:C19H18F3N3O2
- Molecular Weight:377.36

Sheng, Xiaohong; Sheng, Xiaoxia; Jiang, Xiawei. Preparation of crystalline form of lasmiditan and its pharmaceutical composition. Assignee SoliPharma LLC, Peop. Rep. China. WO 2018010345. (2018).
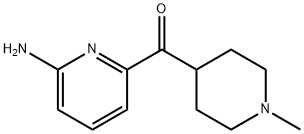
613678-03-8
63 suppliers
inquiry
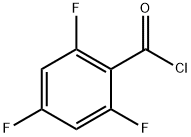
79538-29-7
172 suppliers
$12.00/1g

439239-90-4
147 suppliers
$49.00/10mg
Yield:439239-90-4 100%
Reaction Conditions:
in 1,4-dioxane for 3 h;Product distribution / selectivity;Heating / reflux;
Steps:
8
Combine 2-amino-6-(l-methylpiperidin-4-ylcarbonyl) pyridine (0.20 g, 0.92 mmol), 2,4, 6-Trifluorobenzoyl chloride (0.357 g, 1.84 mmol), and 1, 4-Dioxane (10 mL), and stir while heating at reflux. After 3 hr. , cool the reaction mixture to ambient temperature and concentrate. Load the concentrated mixture onto an SCX column (lOg), wash with methanol, and elute with 2M ammonia in methanol. Concentrate the eluent to obtain the free base of the title compound as an oil (0.365 g (>100%)). Dissolve the oil in methanol (5 mL) and treat with ammonium chloride (0.05 g, 0.92 mmol). Concentrate the mixture and dry under vacuum to obtain the title compound. HRMS Obs. m/z 378.1435, Calc. m/z 378.1429 ; m. p. 255°C (dec).
References:
ELI LILLY AND COMPANY WO2003/84949, 2003, A1 Location in patent:Page/Page column 35

68947-43-3
165 suppliers
$10.00/1g

439239-90-4
147 suppliers
$49.00/10mg

19798-81-3
448 suppliers
$5.00/1g

439239-90-4
147 suppliers
$49.00/10mg

79538-29-7
172 suppliers
$12.00/1g

439239-90-4
147 suppliers
$49.00/10mg
28314-80-9
316 suppliers
$6.00/1g

439239-90-4
147 suppliers
$49.00/10mg

