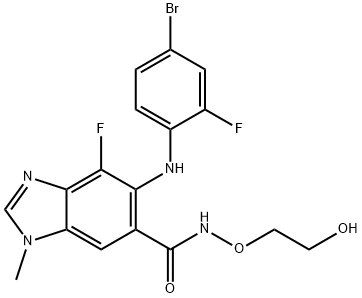
Binimetinib synthesis
- Product Name:Binimetinib
- CAS Number:606143-89-9
- Molecular formula:C17H15BrF2N4O3
- Molecular Weight:441.23
Anon. Process for the preparation of 5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-1-methyl-1H-benz[d]imidazole-6-carboxylic acid. IP.com Journal. Volume 15. Issue 12B. Pages 1pp.Journal; Patent. (2015).
105931-73-5
370 suppliers
$5.00/5g

606143-89-9
198 suppliers
$45.00/5mg
Yield:-
Steps:
Multi-step reaction with 5 steps
1: caesium carbonate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene / toluene; 1,4-dioxane / 8 h / 99 °C / Inert atmosphere
2: potassium trimethylsilonate / N,N-dimethyl-formamide; tetrahydrofuran / 1.67 h / 20 - 25 °C / Inert atmosphere
3: 1,1'-carbonyldiimidazole / tetrahydrofuran / 2 h / 20 - 50 °C
4: N,N-dimethyl-formamide; tetrahydrofuran / 1 h / 50 °C / Inert atmosphere
5: phosphoric acid; water / acetonitrile / 6.25 h / 20 - 53 °C
References:
NOVARTIS AG;ARRAY BIOPHARMA INC.;KRELL, Christoph, Max;MISUN, Marian;NIEDERER, Daniel, Andreas;PACHINGER, Werner, Heinz;WOLF, Marie-christine;ZIMMERMANN, Daniel;LIU, Weidong;STENGEL, Peter, J.;NICHOLS, Paul WO2014/63024, 2014, A1
![5-((4-bromo-2-fluorophenyl)amino)-N-(2-(tert-butoxy)ethoxy)-4-fluoro-1-methyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20200119/GIF/1604812-70-5.gif)
1604812-70-5
10 suppliers
inquiry

606143-89-9
198 suppliers
$45.00/5mg
![5-((4-broMo-2-fluorophenyl)aMino)-4-fluoro-1-Methyl-1H-benzo[d]iMidazole-6-carboxylic acid](/CAS/20130318/GIF/CB32627101.gif)
1415564-99-6
24 suppliers
inquiry

606143-89-9
198 suppliers
$45.00/5mg
![Methyl 5-aMino-4-fluoro-1-Methyl-1H-benzo[d]iMidazole-6-carboxylate](/CAS/GIF/918321-20-7.gif)
918321-20-7
84 suppliers
$13.00/100mg

606143-89-9
198 suppliers
$45.00/5mg
![methyl 5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-1H-benzo[d]imidazole-6-carboxylate](/CAS/20180601/GIF/606143-48-0.gif)
606143-48-0
24 suppliers
inquiry

606143-89-9
198 suppliers
$45.00/5mg


