
Iodopyrazine synthesis
- Product Name:Iodopyrazine
- CAS Number:32111-21-0
- Molecular formula:C4H3IN2
- Molecular Weight:205.98

14508-49-7

32111-21-0
General procedure for the synthesis of 2-iodopyrazine from 2-chloropyrazine: a reaction mixture of 2-chloropyrazine (7.5 mL, 83 mmol), NaI (30.3 g, 202 mmol), HOAc (9.6 mL, 168 mmol) and H2SO4 (0.5 mL) in MeCN (105 mL) was heated and refluxed for 4.5 hours. Upon completion of the reaction, the solvent was removed under reduced pressure and water (120 mL) was added. The solution was alkalized with saturated NaHCO3 solution and extracted with dichloromethane (DCM) (2 x 125 mL). The DCM layers were combined, washed sequentially with saturated Na2S2O3 solution and brine, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure to give the crude 2-iodopyrazine as an oil (12.33 g, 20% yield, 71% purity).1H NMR analysis showed that the crude contained less than about 10 mol% of 2-chloropyrazine. Following the same procedure, another batch of 2-chloropyrazine (50 g, 437 mmol) was converted to the crude 2-iodopyrazine (~65 g). The two crude batches were combined and distilled under reduced pressure (ca. 0.75 Torr, boiling point 47 °C) to give 64 g of pure 2-iodopyrazine (60% yield).1H-NMR (CDCl3, 300 MHz) δ 8.40 (dd, J = 1.8, 2.4 Hz, 1H), 8.51 (d, J = 2.4 Hz, 1H), 8.87 (d, J = 1.5 Hz, 1H).

14508-49-7
378 suppliers
$14.00/25g

32111-21-0
168 suppliers
$7.00/1g
Yield:32111-21-0 60%
Reaction Conditions:
Stage #1:2-chloropyrazin with acetic acid;sodium iodide;sulfuric acid in acetonitrile for 4.5 h;Reflux;
Stage #2: with sodium hydrogencarbonate in water
Steps:
1
A reaction mixture of chloropyrazine (7.5 ml, 83 mmol), NaI (30.3 g, 202 15 mmol), HOAc (9.6 ml, 168 mmol) and H2SO4 (0.5 ml) in MeCN (105 ml) was heated at reflux for 4.5 hours. The solvent was removed and water (120 ml) was added. After the solution was basified with saturated NaHCO3, it was extracted with dichloromethane (DCM) (2 x 125 ml). The DCM layers were combined, washed with saturated Na2S2O3, brine and dried. The removal of solvent gave crude iodopyrazine as an oil (12.33 g, 20 71%). Analysis by 1H NMR showed there was less than about 10 mol% of chloropyrazine in the oil. Another batch of chloropyrazine (50 g, 437 mmol) was also converted into crude iodopyrazine (about 65 g) by the same procedure. These two batches of crude iodopyrazine were combined and distillation of the crude iodopyrazine under reduced pressure (about 0.75 torr, bp 47°C) gave pure compound 64 g (60%). 251H-NMR (CDCl3, 300MHz) 8.40 (dd, /=1.8, 2.4Hz, IH), 8.51 (d, /=2.4Hz,lH), 8.87 (d, /=1.5Hz,lH).
References:
INTERNATIONAL PARTNERSHIP FOR MICROBICIDES WO2009/114313, 2009, A2 Location in patent:Page/Page column 15
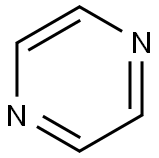
290-37-9
323 suppliers
$5.00/5g

32111-21-0
168 suppliers
$7.00/1g

290-37-9
323 suppliers
$5.00/5g
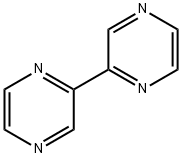
10199-00-5
84 suppliers
$60.00/100mg

32111-21-0
168 suppliers
$7.00/1g
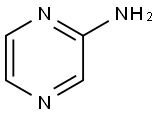
5049-61-6
445 suppliers
$8.00/10g

32111-21-0
168 suppliers
$7.00/1g

290-37-9
323 suppliers
$5.00/5g
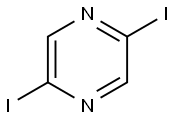
1093418-77-9
26 suppliers
$150.50/50 mg

32111-21-0
168 suppliers
$7.00/1g