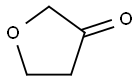
Dihydrofuran-3(2H)-one synthesis
- Product Name:Dihydrofuran-3(2H)-one
- CAS Number:22929-52-8
- Molecular formula:C4H6O2
- Molecular Weight:86.09
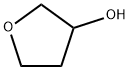
453-20-3

22929-52-8
The general procedure for the synthesis of dihydro-3(2H)-furanone from 3-hydroxytetrahydrofuran was as follows: 3-hydroxytetrahydrofuran (3-OH-THF, 60.6 g, 0.68 mol, 1.0 eq.) was added to a 1 L three-necked flask, followed by the addition of dichloromethane (DCM, 620 mL) and 2,2,6,6-tetramethylpiperidin-1-oxyl radical (TEMPO 1.08 g, 0.0069 mol, 0.01 equiv). The reaction system was cooled to -5°C. Trichloroisocyanuric acid (TCCA, 159.6 g, 0.68 mol, 1.0 eq.) was added in batches while maintaining the temperature at -5°C to 0°C. The reaction mixture was gradually warmed up to room temperature and the reaction progress was monitored by gas chromatography-mass spectrometry (GC-MS). The reaction was completed after about 1 h. The product yield was 95% by GC area percentage analysis.

453-20-3
290 suppliers
$9.00/1g

22929-52-8
259 suppliers
$5.00/250mg
Yield:22929-52-8 95%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;trichloroisocyanuric acid in dichloromethane at -5 - 20; for 1 h;Solvent;
Steps:
7
To a 1 L flask, 3-tetrahydrofuran (3-OH-THF, 60.6 g, 0.68 mol, 1 equ.) was charged, followed by DCM (620 mL) and TEMPO(1.08 g, 0.0069 mol, 0.01 equ.) The solution was cooled to -5 °C. To which TCCA (159.6 g, 0.68 mol, 1 equ.) was added in portions controlling the tern- perature around -5 °C to 0 °C. The resulting mixture was allowed to warm to rt and monitored by GC-MS, Reaction was finished in 1 h to give 95% yield (GC area%).
References:
WO2014/139080,2014,A1 Location in patent:Page/Page column 8; 9
29212-66-6
3 suppliers
inquiry

22929-52-8
259 suppliers
$5.00/250mg

57595-23-0
104 suppliers
$19.00/100mg

22929-52-8
259 suppliers
$5.00/250mg

89898-51-1
64 suppliers
$30.00/100mg

22929-52-8
259 suppliers
$5.00/250mg

927-74-2
425 suppliers
$6.00/5g

22929-52-8
259 suppliers
$5.00/250mg