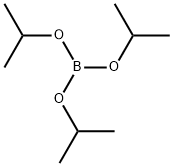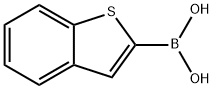
Benzo[b]thien-2-ylboronic acid synthesis
- Product Name:Benzo[b]thien-2-ylboronic acid
- CAS Number:98437-23-1
- Molecular formula:C8H7BO2S
- Molecular Weight:178.02
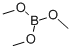
121-43-7
95-15-8
![Benzo[b]thien-2-ylboronic acid](/CAS/GIF/98437-23-1.gif)
98437-23-1
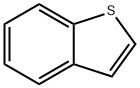
The general procedure for the synthesis of benzothiophene-2-boronic acid from trimethyl borate and benzothiophene was as follows: 144 g (554 mmol) of benzothiophene and 1,000 mL of tetrahydrofuran were added to a 2L reactor and mixed with stirring. After cooling the reaction mixture to -78 °C, 415 mL of n-butyllithium (1.6 M hexane solution) was slowly added dropwise and stirred for 1 hour at this temperature. Subsequently, the temperature of the reaction mixture was gradually brought to room temperature and kept stirring for 12 hours. After cooling again to -78 °C, 80.3 mL (664 mmol) of trimethyl borate was slowly added dropwise and stirred at room temperature for 2 hours. After completion of the reaction, the mixture was cooled to 0°C and acidified by adding 2N aqueous hydrochloric acid. Extraction was separated with ethyl acetate and distilled water and the organic layer was collected. The organic layer was concentrated under reduced pressure and filtered to give 86 g of the intermediate benzothiophene-2-boronic acid in 87.3% yield.
Yield:98437-23-1 87.3%
Reaction Conditions:
Stage #1: Benzo[b]thiophenewith n-butyllithium in tetrahydrofuran at -78 - 20; for 13 h;
Stage #2: Trimethyl borate in tetrahydrofuran at -78 - 20; for 2 h;
Steps:
1.5 Synthesis of intermediate 1-e
Benzothiophene into a 144 g (554 mmol) and 1000 mL of tetrahydrofuran in the 2L reactor and stirred. After cooling to -78 ° C, was added dropwise 415 mL of nbutyllithium(1.6M hexane solution) and stirred for 1 hour. After stirring thetemperature was raised to room temperature for 12 hours, cooled to -78 ° C andwarming up to room temperature and then added dropwise trimethylborate 80.3 mL(664 mmol) was stirred for 2 hours. After cooling to -0 ° C, into a 2N aqueoushydrochloric acid solution and extracted using ethyl acetate and distilled water. Wasstirred into the formed organic layer was concentrated under reduced pressure and hexane and then filtered to give the intermediate 1-e 86 g (yield 87.3%).
References:
KR2016/2328,2016,A Location in patent:Paragraph 0413-0415
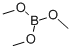
121-43-7
380 suppliers
$14.00/25mL
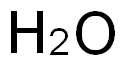
7732-18-5
498 suppliers
$13.50/100ML

95-15-8
384 suppliers
$6.00/5g
![Benzo[b]thien-2-ylboronic acid](/CAS/GIF/98437-23-1.gif)
98437-23-1
299 suppliers
$6.00/1g

95-15-8
384 suppliers
$6.00/5g
![Benzo[b]thien-2-ylboronic acid](/CAS/GIF/98437-23-1.gif)
98437-23-1
299 suppliers
$6.00/1g
![2-BROMOBENZO[B]THIOPHENE](/CAS/GIF/5394-13-8.gif)
5394-13-8
124 suppliers
$26.00/1g
![Benzo[b]thien-2-ylboronic acid](/CAS/GIF/98437-23-1.gif)
98437-23-1
299 suppliers
$6.00/1g