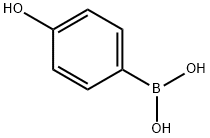
4-Hydroxyphenylboronic acid synthesis
- Product Name:4-Hydroxyphenylboronic acid
- CAS Number:71597-85-8
- Molecular formula:C6H7BO3
- Molecular Weight:137.93
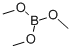
121-43-7
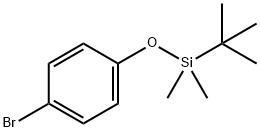
67963-68-2

71597-85-8
1. In a 100 mL three-necked flask, 17 g (0.1 mol) of 4-bromophenol, 16.5 g (0.11 mol) of dimethyl tert-butyl chlorosilane, 30 mL of DMF, 20 mL of triethylamine, and 0.1 g of DMAP were added in this order. the reaction was allowed to run for 24 hours at room temperature. Upon completion of the reaction, equal volumes of water and petroleum ether were added and left to partition, the aqueous phase was discarded. The organic phase was washed to neutrality with water and the solvent was subsequently evaporated to give 1-bromo-4-[(1,1-dimethylethyl)dimethylmethylsilanethoxy]benzene. 2. To a 250 mL three-necked flask were added 2.6 g (0.11 mol) of magnesium fragments, a small amount of crystalline iodine and 50 mL of tetrahydrofuran. Subsequently, 28.7 g (0.1 mol) of a mixture of 1-bromo-4-[(1,1-dimethylethyl)dimethylmethylsilanyloxy]benzene prepared in Step 1 and 20 mL of tetrahydrofuran were added. A small amount of the above mixture was added to initiate the Grignard reaction (if no reaction occurs, it may be heated slightly to initiate the reaction), and then the remaining mixture was added slowly dropwise with stirring, with the dropwise acceleration being controlled to keep the reaction temperature below 35°C. After dropwise addition, stirring is continued for 1 hour at room temperature. 3. The reaction solution was cooled to -65 °C via an acetone-liquid nitrogen bath, followed by the slow dropwise addition of 11.44 g (0.11 mol) of trimethyl borate dissolved in 60 mL of tetrahydrofuran at -60 °C. The reaction solution was allowed to cool to -65 °C via an acetone-liquid nitrogen bath. After the dropwise addition, the reaction solution was allowed to warm naturally to -30 °C and then acidified with concentrated hydrochloric acid to pH=1. The reaction solution was transferred to a partition funnel, 100 mL of ethyl acetate was added, and left to partition. The organic phase was washed with saturated saline to neutral and the aqueous phase was separated. 4. The organic phase was transferred to a 250 mL three-necked flask, 28.71 g (0.1 mol) of tetrabutylammonium fluoride was added, and the reaction was carried out at room temperature for 24 hours. Upon completion of the reaction, the mixture was transferred to a dispensing funnel and washed with saturated saline to neutral. After separation of the aqueous phase, the organic phase was evaporated to remove the solvent and recrystallized with petroleum ether to give 4-hydroxyphenylboronic acid as a white solid.
5720-07-0
470 suppliers
$6.00/5g

71597-85-8
333 suppliers
$6.00/1g
Yield:71597-85-8 90.6%
Reaction Conditions:
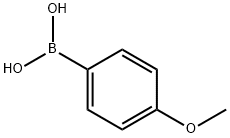
Stage #1:4-methoxyphenylboronic acid with aluminum (III) chloride;acetyl chloride in toluene at 0 - 50; for 2.25 h;Large scale;
Stage #2: with hydrogenchloride in water; pH=3Large scale;
Steps:
1; 1
Use a vacuum pump to evacuate the reactor to -0.1Mpa, reduce the temperature of the reactor to 0, add 1.52kg (10mol) p-methoxyphenylboronic acid, 1kg (12.7mol) acetyl chloride, 12L toluene into the reactor, and mix Stir until it is uniform, then add 1 mol of aluminum chloride. During the reaction, increase the temperature by 10°C every 15 minutes until the temperature reaches 50°C, and then react at a constant temperature for 1h (the methyl chloride gas generated during the reaction is removed to the recovery system by vacuum) To obtain the intermediate compound, add sodium carbonate solution to adjust the pH of the system to 9 to obtain an aqueous layer, continue to use hydrochloric acid to adjust the aqueous layer to pH 3, extract with ethyl acetate, collect the organic layer, concentrate, and recrystallize with heptane 1.25 kg of p-hydroxyphenylboronic acid was obtained, the yield was 90.6%, and the purity was 98.8%.
References:
Business Hezhiji New Materials Technology Center;Lu Zhengxiang CN112047970, 2020, A Location in patent:Paragraph 0038-0039; 0046-047
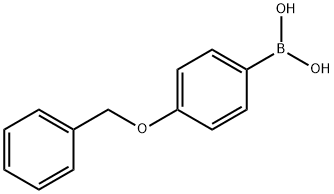
146631-00-7
263 suppliers
$6.00/1g

71597-85-8
333 suppliers
$6.00/1g
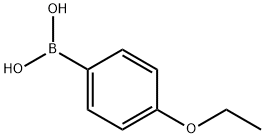
22237-13-4
238 suppliers
$6.00/1g

71597-85-8
333 suppliers
$6.00/1g
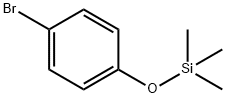
17878-44-3
67 suppliers
$6.00/1g
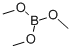
121-43-7
384 suppliers
$13.00/25mL

71597-85-8
333 suppliers
$6.00/1g
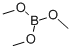
121-43-7
384 suppliers
$13.00/25mL

67963-68-2
135 suppliers
$17.00/1g

71597-85-8
333 suppliers
$6.00/1g