
9-Bromononanoic acid synthesis
- Product Name:9-Bromononanoic acid
- CAS Number:41059-02-3
- Molecular formula:C9H17BrO2
- Molecular Weight:237.13

55362-80-6

41059-02-3
Step 1: Synthesis of 9-bromononanoic acid To an acetone (27 ml) solution of 9-bromo-1-nonanol (1.50 g, 6.72 mmol) cooled to 0°C, saturated NaHCO3 solution (9 ml), NaBr (0.14 g, 1.34 mmol), and 2,2,6,6-tetramethyl-1-piperidinyloxy radical (TEMPO) (0.10 g, 0.67 mmol) were added sequentially. Trichloroisocyanuric acid (3.1 g, 13.44 mmol) was then added in batches. The reaction mixture was stirred at 0 °C for 30 min, then brought to room temperature and continued stirring for 3 h. The reaction was carried out at 0 °C for 2 min. After completion of the reaction, the mixture was cooled to 0 °C, 2-propanol (8 ml) was added slowly and stirring was continued at 0 °C for 30 min. A white precipitate was collected by filtration and the filtrate was concentrated under reduced pressure. H2O (10 ml) and CH2Cl2 (10 ml) were added to the residue to separate the two phases. The aqueous phase was extracted with CH2Cl2 (2 x 10 ml), the organic phases were combined, dried with Na2SO4 and concentrated under reduced pressure to give 1.60 g (yield: 100%) of 9-bromononanoic acid as a white solid.1H NMR (300 MHz, DMSO) δ 3.49 (t, 2H), 2.23-2.08 (m, 2H), 1.84-1.68 (m, 2H) , 1.57-1.14 (m, 10H).

55362-80-6
293 suppliers
$10.00/1g

41059-02-3
177 suppliers
$41.00/1g
Yield:41059-02-3 100%
Reaction Conditions:
with nitric acid at 20 - 80; for 5.5 h;
Steps:
To a solution of concentrated nitric acid (10 mL, 258 mmol) 9-bromononanol (1 gr, 4.48 mmol) was added over a period of 30 minutes, maintaining a temperature of 25-30 0C. The solution was stirred at room temperature for 4 hours, then heated to 80 0C and stirred for an additional hour. The reaction mixture was then cooled back to room temperature and diluted carefully with 100 mL of distilled water. The product was extracted with diethyl ether (4x25 mL) after which the organic phases where combined and dried over magnesium sulfate. The mixture was then filtered and concentrated in vacuo to yield product 4a quantitatively. 1H-NMR (200 MHz, CDCl3): 1.3-1.5 (m; 8H), 1.59-1.71 (m; 2H), 1.78-1.92 (m; 2H), 2.36 (t; / = 7.4 Hz ;2H), 3.40 (t; /= 6.8 Hz; 2H), 9.8 (m, IH).
References:
NATIONAL INSTITUTE FOR BIOTECHNOLOGY IN THE NEGEV;MEIJLER, Michael M.;AMARA, Neri;RAYO, Josep WO2011/1419, 2011, A1 Location in patent:Page/Page column 17

6008-27-1
3 suppliers
inquiry

41059-02-3
177 suppliers
$41.00/1g

3937-56-2
338 suppliers
$9.00/10g

41059-02-3
177 suppliers
$41.00/1g

3788-56-5
96 suppliers
$22.00/100mg

41059-02-3
177 suppliers
$41.00/1g
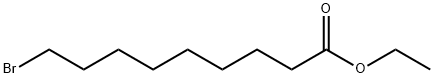
28598-81-4
99 suppliers
$197.00/1g

41059-02-3
177 suppliers
$41.00/1g
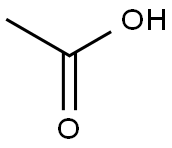
64-19-7
1601 suppliers
$10.00/25ML