
9-Bromo-1-nonanol synthesis
- Product Name:9-Bromo-1-nonanol
- CAS Number:55362-80-6
- Molecular formula:C9H19BrO
- Molecular Weight:223.15

3937-56-2

55362-80-6
1,9-Nonanediol (20 g, 0.125 mol) was dissolved in toluene (500 mL) in a 500 mL round bottom flask fitted with a Dean-Stark water separator. Hydrobromic acid (48%, 21 mL, 188 mmol) was slowly added to this solution, followed by heating and refluxing the reaction mixture for 30 h, during which time the resulting water was removed by means of a Dean-Stark water separator. The progress of the reaction was monitored by thin layer chromatography (TLC) to confirm complete consumption of 1,9-nonanediol. Upon completion of the reaction, the mixture was cooled to room temperature and washed sequentially with 1 M hydrochloric acid (100 mL), 1 M sodium hydroxide solution (100 mL), water (100 mL) and saturated saline (100 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by fractional distillation to give 9-bromo-1-nonanol in 94% yield. Boiling point: 124-128°C/2 mmHg (literature value: 125-126°C/2 mmHg).

3937-56-2
338 suppliers
$9.00/10g

55362-80-6
293 suppliers
$10.00/1g
Yield:55362-80-6 95%
Reaction Conditions:
with hydrogen bromide in toluene; for 7.5 h;Reflux;Time;
Steps:
2.2.1; 2.2.2 Synthesis of compound 2
Dissolve 1,9-nonanediol (40g, 0.25mol) in 350mL anhydrous toluene, and add 40% hydrobromic acid (60.68g, 0.3mol) to the solution, and heat and reflux for 7.5 hours. Cooled to room temperature, quenched with saturated sodium bicarbonate, extracted, combined the organic phases, washed with saturated brine three times, dried over anhydrous magnesium sulfate and concentrated. The crude product was purified by column chromatography to obtain 52.7g of pure product The yield is 95%.
References:
CN112299991,2021,A Location in patent:Paragraph 0025; 0049-0051; 0061-0062
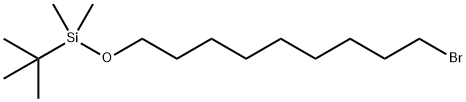
149051-24-1
18 suppliers
inquiry

55362-80-6
293 suppliers
$10.00/1g
silylene]bis-](/CAS/20210305/GIF/172294-15-4.gif)
172294-15-4
0 suppliers
inquiry

55362-80-6
293 suppliers
$10.00/1g

41059-02-3
177 suppliers
$35.00/1g

55362-80-6
293 suppliers
$10.00/1g

53596-82-0
11 suppliers
inquiry

55362-80-6
293 suppliers
$10.00/1g