
2-(Chloromethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(Chloromethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:83622-42-8
- Molecular formula:C7H14BClO2
- Molecular Weight:176.45
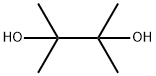
76-09-5

83622-42-8
The general procedure for the synthesis of 2-(chloromethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane from pinacol was as follows: to a mixed solution containing triisopropylborate (15 mL, 65 mmol), chloroiodomethane (13 g, 72 mmol), and tetrahydrofuran (78 mL) was added slowly and dropwise at -78 °C (external temperature) n-butyllithium ( 1.6 M hexane solution, 41 mL, 65 mmol) at -78 °C (external temperature), and the dropwise addition lasted for 20 min. After the dropwise addition, the reaction mixture was warmed to room temperature and stirred for 2.5 hours. Subsequently, the reaction mixture was cooled to 0 °C (external temperature) and a 4N hydrochloric acid-ethyl acetate solution was slowly added dropwise at this temperature until the reaction mixture reached neutrality. Pinacol (7.7 g, 65 mmol) was added to the reaction mixture while maintaining 0 °C, then the mixture was warmed to room temperature and stirred for 40 min. Upon completion of the reaction, the solvent was removed by evaporation under reduced pressure and the resulting residue was distilled under reduced pressure conditions (63-70 °C, 11 mmHg) to afford finally 2-(chloromethyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (9.2 g, 52 mmol, 81% yield). The product was characterized by 1H-NMR (CDCl3) with chemical shift δ (ppm) of 1.30 (12H, s), 2.97 (2H, s).

76-09-5
396 suppliers
$8.19/250mg

83622-42-8
81 suppliers
$55.00/250mg
Yield: 81%
Reaction Conditions:
Stage #1:Chloroiodomethane with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexane at -78 - 20; for 2.83333 h;
Stage #2:2,3-dimethyl-2,3-butane diol with hydrogenchloride in tetrahydrofuran;hexane;ethyl acetate at 0 - 20; for 0.666667 h;
Steps:
1
To the mixture of triisopropyl borate (15 ml, 65 mmol), chloroiodomethane (13 g, 72 mmol), and tetrahydrofuran (78 ml), n-butyllithium (a 1.6 M n-hexane solution, 41 ml, 65 mmol) was added dropwise at -78° C. (an outer temperature) for 20 min. and then the obtained mixture was stirred at room temperature for 2.5 hours. The reaction mixture was cooled to 0° C. (an outer temperature), and a 4 N hydrochloric acid-ethyl acetate solution was added dropwise thereto at the same temperature until the reaction mixture became neutral. At the same temperature, pinacol (7.7 g, 65 mmol) was added to the reaction mixture, and then the reaction mixture was stirred at room temperature for 40 min. The solvents were evaporated under reduced pressure, and then the obtained residue was distilled under reduced pressure (63-70° C., 11 mmHg), thereby obtaining the entitled compound (9.2 g, 52 mmol, 81%). 1H-NMR Spectrum (CDCl3) δ(ppm): 1.30(12H, s), 2.97(2H, s)
References:
Eisai R&D Management Co., Ltd. US2008/15351, 2008, A1 Location in patent:Page/Page column 11

76-09-5
396 suppliers
$8.19/250mg
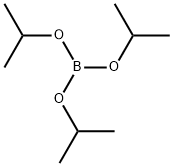
5419-55-6
392 suppliers
$12.00/5g
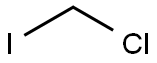
593-71-5
310 suppliers
$6.00/5g

83622-42-8
81 suppliers
$55.00/250mg

593-71-5
310 suppliers
$6.00/5g
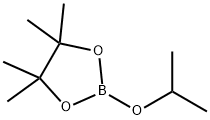
61676-62-8
325 suppliers
$10.00/5g

83622-42-8
81 suppliers
$55.00/250mg

76-09-5
396 suppliers
$8.19/250mg

593-71-5
310 suppliers
$6.00/5g

83622-42-8
81 suppliers
$55.00/250mg

74-97-5
0 suppliers
$15.00/25 g

61676-62-8
325 suppliers
$10.00/5g

83622-42-8
81 suppliers
$55.00/250mg