
3-Fluoro-2-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzonitrile synthesis
- Product Name:3-Fluoro-2-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzonitrile
- CAS Number:624741-47-5
- Molecular formula:C13H15BFNO2
- Molecular Weight:247.07
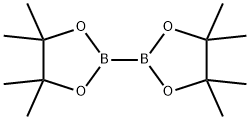
73183-34-3
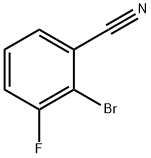
425379-16-4

624741-47-5
2-Bromo-3-fluorobenzonitrile (1.20 g, 6.00 mmol), dried potassium acetate (1.18 g, 12.0 mmol) and bis(pinacolato)diboron (1.75 g, 6.89 mmol) were mixed in 1,4-dioxane (14.7 ml). The mixture was degassed by nitrogen bubbling for 50 min, followed by addition of dimethyl sulfoxide (0.3 ml). Dichloro[1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichloromethane adduct (0.1481 g, 0.181 mmol) was then added and again degassed for 10 min. The reaction mixture was heated at 90°C for 18 hours in a nitrogen atmosphere. After completion of the reaction, it was cooled to room temperature, filtered through glass fiber paper and the solids were washed with a small amount of dichloromethane. The filtrates were combined and concentrated by vacuum evaporation. The residue was partitioned between 2M NaOH aqueous solution (21 ml) and ether (20 ml). The aqueous layer was acidified to pH 5 with concentrated hydrochloric acid (3 ml) to induce precipitation of the solid. The mixture was allowed to stand in a refrigerator for 3 days, the precipitate was collected by filtration, washed with water and dried in vacuum to give 6-cyano-2-fluorophenylboronic acid pinacol ester (0.9191 g, 62%) as a white solid. The product was characterized by 1H NMR (360 MHz, DMSO-d6): δ 1.34 (12H, s), 7.55-7.59 (1H, m), 7.69-7.75 (2H, m).

73183-34-3
587 suppliers
$6.00/5g

425379-16-4
116 suppliers
inquiry

624741-47-5
48 suppliers
$28.00/100mg
Yield:624741-47-5 62%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane;dimethyl sulfoxide at 90; for 18 h;
Steps:
2.a
A mixture of 2-bromo-3-fluorobenzonitrile (1.20 g, 6.00 mmol), dried potassium acetate (1.18 g, 12.0 mmol) and bis (pinacolato) diboron (1.75 g, 6.89 mmol) in 1, 4-dioxan (14.7 ml) and dimethylsulfoxide (0.3 ml) was degassed by bubbling nitrogen through the mixture for 50 min. Dichloro [l, 1'-bis (diphenylphosphino) ferrocene] palladium (II) dichloromethane adduct (0.1481 g, 0.181 mmol) was added and the mixture was degassed for a further 10 min, then heated at 90°C under nitrogen for 18 h. After allowing to cool, the mixture was filtered through glass fibre paper, and the solid was washed with a little dichloromethane. The combined filtrates were evaporated in vacuo and the residue was partitioned between 2 M aqueous NaOH (21 ml) and diethyl ether (20 ml). The aqueous layer was then acidified to pH 5 with concentrated hydrochloric acid (3 ml), causing a solid to precipitate. After leaving in a fridge for 3 days, the solid was collected by filtration, washed with water and dried under vacuum to leave 0.9191 g (62%) of the title compound as a white solid: 1H NMR (360 MHz, DMSO-d6) 8 1.34 (12H, s), 7.55-7. 59 (1H, m), 7.69-7. 75 (2H, m).
References:
WO2003/93272,2003,A1 Location in patent:Page/Page column 37