
7-Fluoro-1-indanone synthesis
- Product Name:7-Fluoro-1-indanone
- CAS Number:651735-59-0
- Molecular formula:C9H7FO
- Molecular Weight:150.15
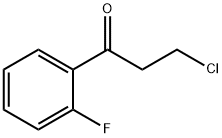
898767-04-9

651735-59-0
The general procedure for the synthesis of 7-fluoro-1-indanone from 3-chloro-1-(2-fluorophenyl)propan-1-one is as follows: first, a mixture of SOCl2 (1.5 equiv.) and substituted benzoic acid in benzene is refluxed until no gas escapes. After cooling to room temperature, the mixture was concentrated using a rotary evaporator. The concentrate was dissolved in dichloroethane and a dichloroethane solution of AlCl3 (1.0 eq.) was slowly added at 10-20 °C. Subsequently, ethylene gas was passed into the reaction system for 4 hours, after which the mixture was stirred overnight and the reaction was quenched with 4N HCl. The organic and aqueous layers were separated and the aqueous layer was extracted with Et2O (3 x 250 mL). The organic extracts were combined and washed sequentially with H2O (3 × 150 mL), saturated NaHCO3 (3 × 150 mL) and brine (1 × 150 mL), dried over MgSO4 and concentrated. The concentrate was mixed with a slurry of AlCl3 (9.0 g, 10 eq.) and NaCl (2.4 g, 6 eq.) and stirred at 180 °C for 2 hours. Alternatively, the concentrate was mixed with concentrated sulfuric acid and stirred at 85°C for 1 hour. Upon completion of the reaction, it was cooled to room temperature and ice was slowly added, followed by the addition of concentrated HCl. The reaction mixture was extracted with CH2Cl2 (3 x 500 mL), the organic layers were combined and concentrated, and finally the target product 7-fluoro-1-indanone was purified by column chromatography using hexanes:EtOAc (4:1) as eluent to give the target product 7-fluoro-1-indanone. 1-(2-fluoroethyl)-3-(7-fluoroindan-1-yl)urea was further synthesized from 7-fluoro-1-indanone according to General Method C. The title compound was prepared from commercially available 2-fluorobenzoic acid by General Method E described above. The intermediates 7-fluoro-1-indanone and 7-fluoroindan-1-ylamine were isolated and characterized. 7-fluoro-1-indanone was prepared in 32% yield (6.85 g from 20.00 g, 0.14 mol of 2-fluorobenzoic acid) using SOCl2 (15.60 mL, 0.21 mol), AlCl3 (19.00 g, 0.14 mol), additional AlCl3 (285.50 g, 2.14 mol) and NaCl (75.10 g, 1.29 mol). The spectral data were as follows: 1H NMR (300 MHz, CDCl3) δ 2.67-2.80 (m, 2H), 3.2 (t, 2H, J = 5.9 Hz), 7.0 (t, 1H, J = 8.5 Hz), 7.3 (d, 1H, J = 7.6 Hz), 7.6 (m, 1H).

898767-04-9
55 suppliers
$31.00/100mg

651735-59-0
145 suppliers
$12.00/100mg
Yield:651735-59-0 32%
Reaction Conditions:
with aluminum (III) chloride;sodium chloride at 130 - 180; for 2 h;
Steps:
General Procedure E for the Synthesis of Fluoroethyl Substituted Indan Ureas A mixture of SOCl2 (1.5 eq) and substituted benzoic acids in benzene was refluxed until no more gas evolution was observed. After cooling to room temperature the mixture was concentrated on a rotary evaporator. The concentrate was taken up in dichloroethane and added to a solution of AlCl3 (1.0 eq) in dichloroethane at 10-20° C. Ethylene was bubbled for 4 hours after which the resulting mixture was stirred overnight and quenched into 4 N HCl. The resulting layers were separated and the aqueous layer was extracted with Et2O (3×250 mL). The combined organic extracts were washed with H2O (3×150 mL), saturated NaHCO3 (3×150 mL), brine (1×150 mL), dried over MgSO4 and concentrated. The concentrate was added to a slurry of AlCl3 (9.0 g, 10 eq) and NaCl (2.4 g, 6 eq) at 130° C. The resulting mixture was stirred at 180° C. for 2 hours. Alternatively, this concentrate was mixed with concentrated sulfuric acid and the resulting mixture was stirred at 85° C. one hour. The reaction mixture was cooled to room temperature and ice was slowly added, followed by concentrated HCl. The resulting mixture was extracted with CH2Cl2 (3×500 mL) and the combined organic extracts were concentrated and purified by column chromatography using hexane:EtOAc (4:1) as eluant to give the desired substituted indanonesxix. Thus, the title fluoroethyl ureas were obtained from these indanones according the protocol described in general procedure C.Synthesis of 1-(2-fluoro-ethyl)-3-(7-fluoro-indan-1-yl)-ureaThe title compound was generated from commercially available 2-fluororobenzoic acid according to the general procedure E described above. The intermediates 7-fluoro-1-indanone and 7-fluoro-indan-1-ylamine were isolated and characterized.7-Fluoro-1-indanone: 6.85 g (32%) of the title indanone was obtained from 2-fluoro-benzoic acid (20.00 g, 0.14 mol), SOCl2(15.60 mL, 0.21 mol), AlCl3 (19.00 g, 0.14 mol), an additional AlCl3 (285.50 g, 2.14 mol) and NaCl (75.10 g, 1.29 mol) according to the protocols as outlined in general procedure E above. Spectroscopic data: 1H NMR (300 MHz, CDCl3) δ 2.67-2.80 (m, 2H), 3.2 (t, 2H, J=5.9 Hz), 7.0 (t, 1H, J=8.5 Hz), 7.3 (d, 1H, J=7.6 Hz), 7.6 (m, 1H).
References:
US2008/255239,2008,A1 Location in patent:Page/Page column 22; 23

628732-03-6
70 suppliers
$66.00/100mg

651735-59-0
145 suppliers
$12.00/100mg
71095-56-2
4 suppliers
inquiry

651735-59-0
145 suppliers
$12.00/100mg

74-85-1
113 suppliers
$79.00/11l
393-52-2
397 suppliers
$10.00/25g

651735-59-0
145 suppliers
$12.00/100mg
445-29-4
432 suppliers
$5.00/10g

651735-59-0
145 suppliers
$12.00/100mg