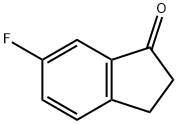
6-Fluoro-1-indanone synthesis
- Product Name:6-Fluoro-1-indanone
- CAS Number:1481-32-9
- Molecular formula:C9H7FO
- Molecular Weight:150.15

772-70-3

1481-32-9
The general procedure for the synthesis of 6-fluoro-1-indanone from 4-fluoro-benzenepropanoyl chloride was as follows: aluminum trichloride (AlCl3, 27.8 g, 208 mmol) was suspended in 200 mL of 1,2-dichloroethane. The mixture was cooled to 0-5 °C under nitrogen protection and a solution of 4-fluoro-phenylpropionyl chloride (27.75 g, 148.8 mmol) dissolved in 140 mL of 1,2-dichloroethane was added slowly and dropwise over a period of 1 hour. After removal of the cooling bath, stirring was continued for 30 minutes, followed by 2 hours of reaction at 70°C. Upon completion of the reaction, the mixture was cooled to room temperature and poured into a mixture of ice and 330 mL of concentrated hydrochloric acid (36-38%). The aqueous layer was extracted with dichloromethane (CH2Cl2) and the combined organic layers were washed sequentially with water (2×), 5% sodium bicarbonate (NaHCO3) solution and saturated saline. The organic layer was dried over anhydrous magnesium sulfate (MgSO4), filtered to remove the desiccant, and the solvent was evaporated under reduced pressure to give 19.02 g of 6-fluoro-1-indanone in 85% yield.

772-70-3
10 suppliers
$90.00/100mg

1481-32-9
238 suppliers
$5.00/250mg
Yield:1481-32-9 85%
Reaction Conditions:
with aluminum (III) chloride in 1,2-dichloro-ethane at 0 - 70; for 3.5 h;
Steps:
45-50.ii
AlCl3 (27.8 g, 208 mmol) was suspended in 200 ml 1,2-dichloroethane. The mixture was cooled under a nitrogen atmosphere to 0-5° C. and a solution of the acid chloride (27.75 g, 148.8 mmol) in 140 ml 1,2-dichloroethane was added dropwise in 1 h. The cooling bath was removed and after stirring for 30 min., stirring was continued for 2 hours at 70° C. After cooling to room temperature the reaction mixture was poured into a mixture of ice and 330 ml concentrated HCl (36-38%). The aqueous layer was extracted with CH2Cl2 and the resulting organic layer was washed with H2O (2×), 5% NaHCO3 and brine. The organic layer was dried (MgSO4). The drying agent was removed by filtration and the solvent by evaporation under reduced pressure to give 19.02 g (85%).
References:
SOLVAY PHARMACEUTICALS B.V. US2006/122189, 2006, A1 Location in patent:Page/Page column 27

459-31-4
231 suppliers
$6.00/250mg

1481-32-9
238 suppliers
$5.00/250mg

1228180-96-8
3 suppliers
inquiry

1481-32-9
238 suppliers
$5.00/250mg
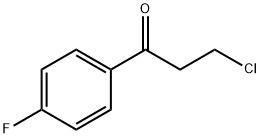
347-93-3
186 suppliers
$8.00/5g

1481-32-9
238 suppliers
$5.00/250mg
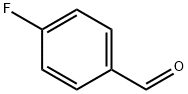
459-57-4
603 suppliers
$6.00/25g

1481-32-9
238 suppliers
$5.00/250mg