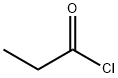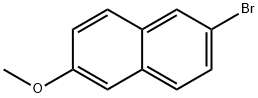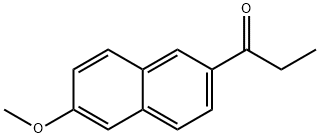
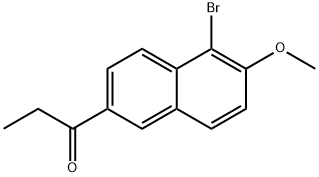
6-Methoxy-2-Acetyl Naphthalene synthesis
- Product Name:6-Methoxy-2-Acetyl Naphthalene
- CAS Number:2700-47-2
- Molecular formula:C14H14O2
- Molecular Weight:214.26
92189-66-7

2700-47-2
Example 10 Synthesis of 1-(6-methoxynaphthalen-2-yl)propan-1-one: 29.3 g of 5-bromo-6-methoxy-2-propionyl naphthalene was dissolved in 100 ml of anhydrous dichloromethane and 20.8 ml of homotrimethylbenzene. Under strong stirring, 20 g of anhydrous aluminum chloride was added in batches to the reaction mixture that was cooled to -5 °C while the temperature was controlled below 20 °C. The reaction mixture was continued to be stirred at room temperature for 3 hours. Subsequently, the reaction mixture was slowly poured under strong stirring into a mixture consisting of 110 g of ice and 35 ml of a 35% (w/v) aqueous hydrochloric acid solution. After stirring for 15 minutes, the organic and aqueous layers were separated and the aqueous layer was discarded. The organic layer was sequentially washed twice with 50 ml of aqueous 6N hydrochloric acid solution and then with 50 ml of water. Next, 50 ml of water was added to the organic solution and the pH was adjusted with 30% (w/v) aqueous sodium hydroxide to 12. After discarding the aqueous phase, the organic phase was dried with anhydrous sodium sulfate and subsequently evaporated to dryness under vacuum. The residue was crystallized by n-heptane to give 20.2 g of 1-(6-methoxynaphthalen-2-yl)propan-1-one in 94.3% yield.

93-04-9
472 suppliers
$13.00/25g
79-05-0
224 suppliers
$6.00/25g

2700-47-2
133 suppliers
$5.00/250mg
Yield: 85%
Reaction Conditions:
with triethylamine;tin(ll) chloride at 5 - 19; for 32.3333 h;Concentration;Temperature;
Steps:
3 Example 3
In the is provided with an agitator, reflux condenser, thermometer, dropping funnel in the reaction container, adding triethylamine to 310 ml, stannous chloride 0.65mol, control the stirring speed 190rpm, reducing the temperature of the solution to 5 °C, β-naphthyl methyl ether is slowly added (2) 0.65mol, adds by drops third amide (3) 0.47mol, dropping time control, in 80 min, maintaining the temperature of the solution reaction 9h, raising the temperature of the solution to 19 °C, standing 22h, the reactants are poured into the 1.8L the mass fraction of 25% sodium bisulfite solution, control the temperature of the solution in the 5 °C, steam distillation, until completely distilled until the triethylamine, control the stirring speed 130rpm, let solution of natural cooling, solidifying oily substance, filtering, potassium nitrate solution, anhydrous calcium sulfate dehydration, in the mass fraction is 92% recrystallized in cyclohexane solution, to get the yellow solid 6-methoxy-2-c acyl-naphthaline 85.49g, yield 85%.
References:
Chengdu Cardiff Technology Co., Ltd.;Liao, Ruer CN105439833, 2016, A Location in patent:Paragraph 0015; 0016

92189-66-7
13 suppliers
inquiry

2700-47-2
133 suppliers
$5.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2700-47-2
133 suppliers
$5.00/250mg