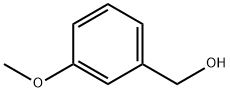
m-Anisyl alcohol synthesis
- Product Name:m-Anisyl alcohol
- CAS Number:6971-51-3
- Molecular formula:C8H10O2
- Molecular Weight:138.16
![Benzene, 1-methoxy-3-[[(trimethylsilyl)oxy]methyl]-](/CAS/20210305/GIF/132816-03-6.gif)
132816-03-6

6971-51-3
The general procedure for the synthesis of m-methoxybenzyl alcohol using the compound (CAS:132816-03-6) as starting material was as follows: the substrate (1 mmol) was mixed with PEG-SANM nanocomplex (8 mg) in methanol (2 mL) and the reaction was stirred at room temperature. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the catalyst was removed by filtration and the solvent was removed by distillation under reduced pressure. The crude product was purified by silica gel column chromatography, resulting in pure m-methoxybenzyl alcohol and by-product phenol.
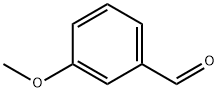
591-31-1
430 suppliers
$10.00/5g

6971-51-3
297 suppliers
$8.00/5g
Yield:6971-51-3 100%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 20; for 1 h;
Steps:
Step-I (Preparation of benzyl alcohols)
General procedure: benzaldehyde derivatives (1 mmol) in methanol (15 mL), sodium borohydride (2 mmol) and stirred at room temperature for 1 h. Usual work-up, quantitative yield.
References:
Parihar, Swati;Kumar, Amit;Chaturvedi, Amit K.;Sachan, Naresh Kumar;Luqman, Suaib;Changkija, Bendangla;Manohar, Murli;Prakash, Om;Chanda;Khan, Feroz;Chanotiya;Shanker, Karuna;Dwivedi, Anila;Konwar, Rituraj;Negi, Arvind S. [Journal of Steroid Biochemistry and Molecular Biology,2013,vol. 137,p. 332 - 344]
![Benzene, 1-methoxy-3-[[(trimethylsilyl)oxy]methyl]-](/CAS/20210305/GIF/132816-03-6.gif)
132816-03-6
0 suppliers
inquiry

6971-51-3
297 suppliers
$8.00/5g
5368-81-0
145 suppliers
$6.00/1g

6971-51-3
297 suppliers
$8.00/5g

591-31-1
430 suppliers
$10.00/5g

6971-51-3
297 suppliers
$8.00/5g

24318-46-5
1 suppliers
inquiry
586-38-9
399 suppliers
$5.00/5g

498-00-0
346 suppliers
$21.00/25g

6971-51-3
297 suppliers
$8.00/5g