
(naphthalene-1-yl)-{4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2- synthesis
- Product Name:(naphthalene-1-yl)-{4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-
- CAS Number:528610-01-7
- Molecular formula:C28H28BNO2
- Molecular Weight:421.34
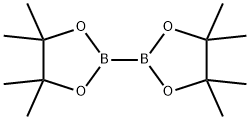
73183-34-3
591 suppliers
$6.00/5g

138310-84-6
108 suppliers
$27.00/200mg

528610-01-7
22 suppliers
inquiry
Yield:528610-01-7 97%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in toluene at 100;Inert atmosphere;
Steps:
1 N-phenyl-N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)naphthalen-1-amine (Compound 5)
Compound 5 [Shin, D.; Paek, W.; Choi, B.; Kwon, O.; Kim, M.; Son, Y.; Han, E.; Song, J. U.S. Pat. Appl. Publ., 20070276160, 29 Nov. 2007] was prepared as follows: a mixture of Compound 4 (2.54 g, 6.79 mmol), bis(pinacolato)diboron (3.62 g, 14.3 mmol), [1,1′-bis(diphenylphosphino)-ferrocene]dichloropalladium(II) (0.298 g, 0.407 mmol), potassium acetate (2.00 g, 20.4 mmol) and anhydrous toluene (50 mL) was degassed with argon for about 28 min while stirring. The reaction mixture was then maintained under argon at about 100° C. while stirring. Upon confirming consumption of the starting material by TLC (SiO2, 4:1 hexanes-CH2Cl2), the reaction was cooled to RT and poured over EtOAc (ca. 200 mL). The organics were then washed with sat. NaHCO3, water and brine, dried over MgSO4, filtered and concentrated in vacuo. Purification of the crude product via flash chromatography (SiO2, 2:1 to 3:2-hexanes: CH2Cl2 to 100% CH2Cl2) yielded Compound 5 (2.78 g, 97%) as a colorless foam: confirmed by LCMS (APCI): calculated for C28H29BNO2 (M+H+): 422; Found: 422.
References:
US9112159,2015,B2 Location in patent:Page/Page column 44-45
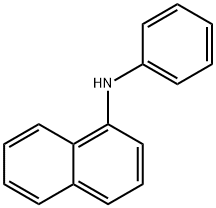
90-30-2
331 suppliers
$10.00/25g

528610-01-7
22 suppliers
inquiry
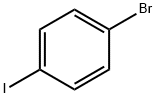
589-87-7
540 suppliers
$10.00/1g

528610-01-7
22 suppliers
inquiry